Mesenchymal precursor cells in the blood of normal individuals
- PMID: 11056678
- PMCID: PMC17820
- DOI: 10.1186/ar130
Mesenchymal precursor cells in the blood of normal individuals
Abstract
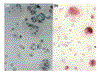
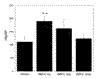
STATEMENT OF FINDINGS: Mesenchymal precursor cells found in the blood (BMPCs) of normal persons adhere to plastic and glass and proliferate logarithmically in DMEM-20% fetal calf serum (FCS) without growth factors. They form cells with fibroblast-like and stromal morphology, which is not affected by eliminating CD34, CD3, or CD14 cells. Osteogenic supplements (dexamethasone, ascorbic acid, and beta-glycerophosphate) added to the culture inhibited fibroblast formation, and BMPCs assumed the cuboidal shape of osteoblasts. After 5 days in supplemented medium, the elutriated cells displayed alkaline phosphatase (AP), and the addition of bone morphogenetic protein (BMP)2 (1 ng) doubled AP production (P < 0.04). Two weeks later, 30% of the cells were very large and reacted with anti-osteocalcin antibody. The same cultures also contained sudanophlic adipocytes and multinucleated giant cells that stained for tartrate-resistant acid phosphatase (TRAP) and vitronectin receptors. Cultured BMPCs immunostain with antibodies to vimentin, type I collagen, and BMP receptors, heterodimeric structures expressed on mesenchymal lineage cells. In addition, BMPCs stain with anti-CD105 (endoglin), a putative marker for bone-marrow mesenchymal stem cells (MSCs).
Figures






References
-
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. - PubMed
-
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Precursor cells of mechanocytes. ExpHematol. 1976;4:267–274. - PubMed
-
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. . J Cell Biochem. 1997;64:278–294. - PubMed
-
- Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun. 1999;265:134–139. - PubMed
-
- Rosen V. Signaling pathways in skeletal formation. A role for BMP receptors. In Ann NY Acad Sci No 785: Molecular and Developmental Biology of Cartilage Edited by de Crombrugghe B. 1996. pp. 56–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

