Estrogen receptor of primary breast cancers: evidence for intracellular proteolysis
- PMID: 11056692
- PMCID: PMC13922
- DOI: 10.1186/bcr92
Estrogen receptor of primary breast cancers: evidence for intracellular proteolysis
Abstract
Iodinated oestradiol-labeled oestrogen receptor (ER) isoforms devoid of amino-terminal ABC domains represent about two-thirds of the whole receptor population detected in cytosol samples from human breast cancers. This high frequency could not be ascribed to the expression of truncated mRNAs, or to the proteolysis of the native ER peptide at the time of homogenization or assay, suggesting an intracellular proteolysis. Free amino-terminal and ligand-binding domains maintained together within oligomeric structure(s); increase of ionic strength separated them. The amino-terminal region was consistently detected in the cell nucleus by specific immunohistochemistry leading to the concept of a potential intranuclear association between ER cleavage products and/or other regulatory proteins.
Figures



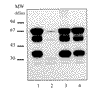

Similar articles
-
Major molecular weight heterogeneity of estrogen receptor from breast cancer is not related to neoplasia.Cancer Biochem Biophys. 1995 Nov;15(2):67-78. Cancer Biochem Biophys. 1995. PMID: 8590437
-
Detection of different estrogen receptor forms in breast cancer cytosol by enzyme immunoassay.Cancer Res. 1997 Mar 15;57(6):1066-72. Cancer Res. 1997. PMID: 9067273
-
Expression and cellular localization of naturally occurring beta estrogen receptors in uterine and mammary cell lines.J Cell Biochem. 2002;86(1):136-44. doi: 10.1002/jcb.10193. J Cell Biochem. 2002. PMID: 12112024
-
[Expression and intranuclear distribution of nucleolin in estrogen receptor-negative and estrogen receptor-positive breast cancers in women measured by laser scanning cytometry].Ann Acad Med Stetin. 2006;52(2):23-32. Ann Acad Med Stetin. 2006. PMID: 17633394 Polish.
-
Abnormal oestrogen receptor in clinical breast cancer.Eur J Cancer. 1992;28(2-3):309-10. doi: 10.1016/s0959-8049(05)80040-1. Eur J Cancer. 1992. PMID: 1591044 Review. No abstract available.
Cited by
-
Targeting Estrogens and Various Estrogen-Related Receptors against Non-Small Cell Lung Cancers: A Perspective.Cancers (Basel). 2021 Dec 24;14(1):80. doi: 10.3390/cancers14010080. Cancers (Basel). 2021. PMID: 35008242 Free PMC article. Review.
-
Expression and clinical implications of estrogen receptors in thoracic malignancies: a narrative review.J Thorac Dis. 2021 Mar;13(3):1851-1863. doi: 10.21037/jtd-20-2277. J Thorac Dis. 2021. PMID: 33841973 Free PMC article. Review.
-
Estrogen-dependent growth and estrogen receptor (ER)-alpha concentration in T47D breast cancer cells are inhibited by VACM-1, a cul 5 gene.Mol Cell Biochem. 2007 Jul;301(1-2):13-20. doi: 10.1007/s11010-006-9392-3. Epub 2006 Dec 22. Mol Cell Biochem. 2007. PMID: 17186378
-
Structural basis for Ca2+-induced activation and dimerization of estrogen receptor α by calmodulin.J Biol Chem. 2012 Mar 16;287(12):9336-44. doi: 10.1074/jbc.M111.334797. Epub 2012 Jan 23. J Biol Chem. 2012. PMID: 22275375 Free PMC article.
-
The Predominant Proteins that React to the MC-20 Estrogen Receptor Alpha Antibody Differ in Molecular Weight between the Mammary Gland and Uterus in the Mouse and Rat.Int J Biomed Sci. 2012 Mar;8(1):51-63. Int J Biomed Sci. 2012. PMID: 23675257 Free PMC article.
References
-
- Heuson JC, Leclercq G, Longeval E, Deboel MC, Mattheiem WH, Heimann R (Edited by McGuire WL, Carbone PP, Volmer EP. New York: Raven Press.) Estrogen receptors: pronostic significance in breast cancer. Estrogen Receptors in Human Breast Cancer. 1975. pp. 57–72.
-
- McGuire WL. Hormone receptors: their role in predicting prognosis and response to endocrine therapy. Semin Oncol. 1978;5:428–433. - PubMed
-
- DeSombre ER, King WJ, Green GL, Jensen EV (Edited by Jordan VC. Wisconsin: University of Wisconsin Press.) Estrogen receptor studies. Laboratory investigations and clinical applications. . Estrogen/Antiestrogen Action and Breast Cancer Therapy. 1986. pp. 303–323.
-
- Leclercq G, Bojar H, Goussard J, Nicholson RI, Pichon MF, Piffanelli A, Pousette A, Thorpe S, Lonsdorfer M. Abbott monoclonal enzyme immunoassay measurement of estrogen receptors in human breast cancer: a european multicenter study. Cancer Res. 1986;46 (Suppl):S4233–S4236. - PubMed
-
- al Saati T, Clamens S, Cohen-Knafo E, Faye JC, Prats H, Coindre JM, Wafflart J, Caveriviere P, Bayard F, Delsol G. Production of monoclonal antibodies to human estrogen-receptor protein (ER) using recombinant ER (RER). Int J Cancer. 1993;55:651–654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

