Increased resistance to acetaminophen hepatotoxicity in mice lacking glutathione S-transferase Pi
- PMID: 11058152
- PMCID: PMC18834
- DOI: 10.1073/pnas.220176997
Increased resistance to acetaminophen hepatotoxicity in mice lacking glutathione S-transferase Pi
Abstract
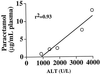
Overdose of acetaminophen, a widely used analgesic drug, can result in severe hepatotoxicity and is often fatal. This toxic reaction is associated with metabolic activation by the P450 system to form a quinoneimine metabolite, N-acetyl-p-benzoquinoneimine (NAPQI), which covalently binds to proteins and other macromolecules to cause cellular damage. At low doses, NAPQI is efficiently detoxified, principally by conjugation with glutathione, a reaction catalyzed in part by the glutathione S-transferases (GST), such as GST Pi. To assess the role of GST in acetaminophen hepatotoxicity, we examined acetaminophen metabolism and liver damage in mice nulled for GstP (GstP1/P2((-/-))). Contrary to our expectations, instead of being more sensitive, GstP null mice were highly resistant to the hepatotoxic effects of this compound. No significant differences between wild-type (GstP1/P2((+/+))) mice and GstP1/P2((-/-)) nulls in either the rate or route of metabolism, particularly to glutathione conjugates, or in the levels of covalent binding of acetaminophen-reactive metabolites to cellular protein were observed. However, although a similar rapid depletion of hepatic reduced glutathione (GSH) was found in both GstP1/P2((+/+)) and GstP1/P2((-/-)) mice, GSH levels only recovered in the GstP1/P2((-/-)) mice. These data demonstrate that GstP does not contribute in vivo to the formation of glutathione conjugates of acetaminophen but plays a novel and unexpected role in the toxicity of this compound. This study identifies new ways in which GST can modulate cellular sensitivity to toxic effects and suggests that the level of GST Pi may be an important and contributing factor in the sensitivity of patients with acetaminophen-induced hepatotoxicity.
Figures


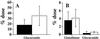

Similar articles
-
Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR.Science. 2002 Oct 11;298(5592):422-4. doi: 10.1126/science.1073502. Science. 2002. PMID: 12376703
-
Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice.Toxicol Appl Pharmacol. 1998 Sep;152(1):193-9. doi: 10.1006/taap.1998.8501. Toxicol Appl Pharmacol. 1998. PMID: 9772215
-
Protective effect of diallyl sulfone against acetaminophen-induced hepatotoxicity in mice.J Biochem Toxicol. 1996;11(1):11-20. doi: 10.1002/(SICI)1522-7146(1996)11:1<11::AID-JBT2>3.0.CO;2-Y. J Biochem Toxicol. 1996. PMID: 8806047
-
Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis.Pharm Res. 2013 Sep;30(9):2174-87. doi: 10.1007/s11095-013-1007-6. Epub 2013 Mar 6. Pharm Res. 2013. PMID: 23462933 Free PMC article. Review.
-
Metabolic phenotyping applied to pre-clinical and clinical studies of acetaminophen metabolism and hepatotoxicity.Drug Metab Rev. 2015 Feb;47(1):29-44. doi: 10.3109/03602532.2014.982865. Epub 2014 Dec 23. Drug Metab Rev. 2015. PMID: 25533740 Review.
Cited by
-
Titanate nanotubes as an efficient oral detoxifying agent against drug overdose: application in rat acetaminophen poisoning.Nanoscale Adv. 2023 Apr 25;5(11):2950-2962. doi: 10.1039/d2na00874b. eCollection 2023 May 30. Nanoscale Adv. 2023. PMID: 37260481 Free PMC article.
-
A systems approach reveals species differences in hepatic stress response capacity.Toxicol Sci. 2023 Oct 30;196(1):112-125. doi: 10.1093/toxsci/kfad085. Toxicol Sci. 2023. PMID: 37647630 Free PMC article.
-
Mice deficient in Cu,Zn-superoxide dismutase are resistant to acetaminophen toxicity.Biochem J. 2006 Nov 1;399(3):455-61. doi: 10.1042/BJ20060784. Biochem J. 2006. PMID: 16831125 Free PMC article.
-
Longitudinal Study of Analgesic Use and Risk of Incident Persistent Tinnitus.J Gen Intern Med. 2022 Nov;37(14):3653-3662. doi: 10.1007/s11606-021-07349-5. Epub 2022 Feb 7. J Gen Intern Med. 2022. PMID: 35132561 Free PMC article.
-
Opportunities and challenges related to saturation of toxicokinetic processes: Implications for risk assessment.Regul Toxicol Pharmacol. 2021 Dec;127:105070. doi: 10.1016/j.yrtph.2021.105070. Epub 2021 Oct 28. Regul Toxicol Pharmacol. 2021. PMID: 34718074 Free PMC article.
References
-
- Villeneuve J P, Raymond G, Bruneau J, Colpron L, Pomier-Layrargues G. Gastroenterol Clin Biol. 1983;7:898–902. - PubMed
-
- Vermeulen N P, Bessems J G, Van de Straat R. Drug Metab Rev. 1992;24:367–407. - PubMed
-
- Anker A L, Smilkstein M J. Conc Controversies Toxicol. 1994;12:335–349. - PubMed
-
- Prescott L F. Paracetamol (Acetaminophen): A Critical Bibliographic Review. London: Taylor & Francis; 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

