Self-organizing biochemical cycles
- PMID: 11058157
- PMCID: PMC18793
- DOI: 10.1073/pnas.220406697
Self-organizing biochemical cycles
Abstract
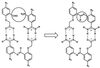
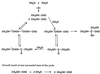
I examine the plausibility of theories that postulate the development of complex chemical organization without requiring the replication of genetic polymers such as RNA. One conclusion is that theories that involve the organization of complex, small-molecule metabolic cycles such as the reductive citric acid cycle on mineral surfaces make unreasonable assumptions about the catalytic properties of minerals and the ability of minerals to organize sequences of disparate reactions. Another conclusion is that data in the Beilstein Handbook of Organic Chemistry that have been claimed to support the hypothesis that the reductive citric acid cycle originated as a self-organized cycle can more plausibly be interpreted in a different way.
Figures

References
-
- Gesteland R F, Cech T R, Atkins J F, editors. The RNA World. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1999.
-
- Miller S L. Science. 1953;117:528–529. - PubMed
-
- Oró J, Kimball A P. Biochem Biophys Res Commun. 1960;2:407–412.
-
- Ferris J P, Sanchez A, Orgel L E. J Mol Biol. 1968;33:693–704. - PubMed
-
- Müller D, Pitsch S, Kittaka A, Wagner E, Wintner C E, Eschenmoser A. Helv Chim Acta. 1990;73:1410–1468.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

