Chimeras of Dictyostelium myosin II head and neck domains with Acanthamoeba or chicken smooth muscle myosin II tail domain have greatly increased and unregulated actin-dependent MgATPase activity
- PMID: 11058169
- PMCID: PMC18802
- DOI: 10.1073/pnas.230441497
Chimeras of Dictyostelium myosin II head and neck domains with Acanthamoeba or chicken smooth muscle myosin II tail domain have greatly increased and unregulated actin-dependent MgATPase activity
Abstract
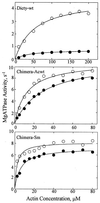
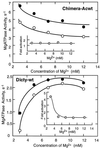
Phosphorylation of the regulatory light chain of Dictyostelium myosin II increases V(max) of its actin-dependent MgATPase activity about 5-fold under normal assay conditions. Under these assay conditions, unphosphorylated chimeric myosins in which the tail domain of the Dictyostelium myosin II heavy chain is replaced by either the tail domain of chicken gizzard smooth muscle or Acanthamoeba myosin II are 20 times more active because of a 10- to 15-fold increase in V(max) and a 2- to 7-fold decrease in apparent K(ATPase) and are only slightly activated by regulatory light chain phosphorylation. Actin-dependent MgATPase activity of the Dictyostelium/Acanthamoeba chimera is not affected by phosphorylation of serine residues in the tail whose phosphorylation completely inactivates wild-type Acanthamoeba myosin II. These results indicate that the actin-dependent MgATPase activity of these myosins involves specific, tightly coupled, interactions between head and tail domains.
Figures






Similar articles
-
Regulation of nonmuscle myosins by heavy chain phosphorylation.J Muscle Res Cell Motil. 2001;22(2):163-73. doi: 10.1023/a:1010552929028. J Muscle Res Cell Motil. 2001. PMID: 11519739 Review.
-
Functional analysis of tail domains of Acanthamoeba myosin IC by characterization of truncation and deletion mutants.J Biol Chem. 2000 Aug 11;275(32):24886-92. doi: 10.1074/jbc.M004287200. J Biol Chem. 2000. PMID: 10840041
-
Tail chimeras of Dictyostelium myosin II support cytokinesis and other myosin II activities but not full development.J Cell Sci. 2002 Nov 15;115(Pt 22):4237-49. doi: 10.1242/jcs.00112. J Cell Sci. 2002. PMID: 12376556
-
Filament structure as an essential factor for regulation of Dictyostelium myosin by regulatory light chain phosphorylation.Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14124-9. doi: 10.1073/pnas.95.24.14124. Proc Natl Acad Sci U S A. 1998. PMID: 9826664 Free PMC article.
-
Phosphorylation of chicken gizzard myosin: myosin filament hypothesis of calcium regulation.Adv Biophys. 1985;19:1-20. doi: 10.1016/0065-227x(85)90049-8. Adv Biophys. 1985. PMID: 2940816 Review.
Cited by
-
Regulation of nonmuscle myosins by heavy chain phosphorylation.J Muscle Res Cell Motil. 2001;22(2):163-73. doi: 10.1023/a:1010552929028. J Muscle Res Cell Motil. 2001. PMID: 11519739 Review.
-
Interacting-heads motif has been conserved as a mechanism of myosin II inhibition since before the origin of animals.Proc Natl Acad Sci U S A. 2018 Feb 27;115(9):E1991-E2000. doi: 10.1073/pnas.1715247115. Epub 2018 Feb 14. Proc Natl Acad Sci U S A. 2018. PMID: 29444861 Free PMC article.
-
Bioinformatics assessment of beta-myosin mutations reveals myosin's high sensitivity to mutations.Trends Cardiovasc Med. 2008 May;18(4):141-9. doi: 10.1016/j.tcm.2008.04.001. Trends Cardiovasc Med. 2008. PMID: 18555187 Free PMC article. Review.
-
Coevolution of head, neck, and tail domains of myosin heavy chains.Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12559-64. doi: 10.1073/pnas.230441597. Proc Natl Acad Sci U S A. 2000. PMID: 11058170 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

