Unprecedented proximal binding of nitric oxide to heme: implications for guanylate cyclase
- PMID: 11060017
- PMCID: PMC305806
- DOI: 10.1093/emboj/19.21.5661
Unprecedented proximal binding of nitric oxide to heme: implications for guanylate cyclase
Abstract
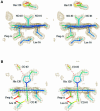
Microbial cytochromes c' contain a 5-coordinate His-ligated heme that forms stable adducts with nitric oxide (NO) and carbon monoxide (CO), but not with dioxygen. We report the 1.95 and 1.35 A resolution crystal structures of the CO- and NO-bound forms of the reduced protein from Alcaligenes xylosoxidans. NO disrupts the His-Fe bond and binds in a novel mode to the proximal face of the heme, giving a 5-coordinate species. In contrast, CO binds 6-coordinate on the distal side. A second CO molecule, not bound to the heme, is located in the proximal pocket. Since the unusual spectroscopic properties of cytochromes c' are shared by soluble guanylate cyclase (sGC), our findings have potential implications for the activation of sGC induced by the binding of NO or CO to the heme domain.
Figures



References
-
- Addison A.W. and Stephanos,J.J. (1986) Nitrosyliron(III) hemoglobin: autoreduction and spectroscopy. Biochemistry, 25, 4104–4113. - PubMed
-
- Brucker E.A., Olson,J.S., Ikeda-Saito,M. and Phillips,G.N.,Jr (1998) Nitric oxide myoglobin: crystal structure and analysis of ligand geometry. Proteins, 30, 352–356. - PubMed
-
- Brünger A.T. (1992) X-PLOR: Version 3.1. A System for X-ray Crystallography and NMR. Yale University Press, New Haven, CT.
-
- Brünger A.T. (1993) Assessment of phase accuracy by cross validation—the free R-value—methods and applications. Acta Crystallogr. D, 49, 24–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

