Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo
- PMID: 11060018
- PMCID: PMC305778
- DOI: 10.1093/emboj/19.21.5672
Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo
Abstract
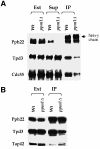
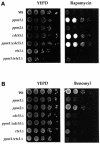
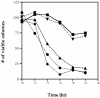
The phosphoprotein phosphatase 2A (PP2A) catalytic subunit contains a methyl ester on its C-terminus, which in mammalian cells is added by a specific carboxyl methyltransferase and removed by a specific carboxyl methylesterase. We have identified genes in yeast that show significant homology to human carboxyl methyltransferase and methylesterase. Extracts of wild-type yeast cells contain carboxyl methyltransferase activity, while extracts of strains deleted for one of the methyltransferase genes, PPM1, lack all activity. Mutation of PPM1 partially disrupts the PP2A holoenzyme in vivo and ppm1 mutations exhibit synthetic lethality with mutations in genes encoding the B or B' regulatory subunit. Inactivation of PPM1 or overexpression of PPE1, the yeast gene homologous to bovine methylesterase, yields phenotypes similar to those observed after inactivation of either regulatory subunit. These phenotypes can be reversed by overexpression of the B regulatory subunit. These results demonstrate that Ppm1 is the sole PP2A methyltransferase in yeast and that its activity is required for the integrity of the PP2A holoenzyme.
Figures





References
-
- Beck T. and Hall,M.N. (1999) The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature, 402, 689–692. - PubMed
-
- Chelsky D., Ruskin,B. and Koshland,D.E.,Jr (1985) Methyl-esterified proteins in a mammalian cell line. Biochemistry, 24, 6651–6658. - PubMed
-
- Chen J., Martin,B.L. and Brautigan,D.L. (1992) Regulation of protein serine–threonine phosphatase type-2A by tyrosine phosphorylation. Science, 257, 1261–1264. - PubMed
-
- Chen J., Parsons,S. and Brautigan,D.L. (1994) Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J. Biol. Chem., 269, 7957–7962. - PubMed
-
- Chen J., Peterson,R.T. and Schreiber,S.L. (1998) α4 associates with protein phosphatases 2A, 4 and 6. Biochem. Biophys. Res. Commun., 247, 827–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

