The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis
- PMID: 11060022
- PMCID: PMC305783
- DOI: 10.1093/emboj/19.21.5711
The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis
Abstract
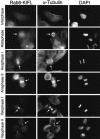
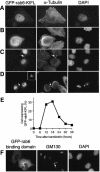
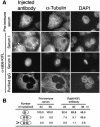
The Rab6-binding kinesin, Rab6-KIFL, was identified in a two-hybrid screen for proteins that interact with Rab6, a small GTPase involved in membrane traffic through the Golgi apparatus. We find that Rab6-KIFL accumulates in mitotic cells where it localizes to the midzone of the spindle during anaphase, and to the cleavage furrow and midbody during telophase. Overexpression of Rab6-KIFL causes a cell division defect resulting in cell death. Microinjection of antibodies to Rab6-KIFL results in the cells becoming binucleate after one cell cycle, and time-lapse microscopy reveals that this is due to a defect in cleavage furrow formation and thus cytokinesis. These data show that endogenous Rab6-KIFL functions in cell division during cleavage furrow formation and cytokinesis, in addition to its previously described role in membrane traffic.
Figures





References
-
- Allan V.J. and Schroer,T.A. (1999) Membrane motors. Curr. Opin. Cell Biol., 11, 476–482. - PubMed
-
- Barr F.A. (1999) A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr. Biol., 9, 381–384. - PubMed
-
- Boman A.L., Kuai,J., Zhu,X., Chen,J., Kuriyama,R. and Kahn,R.A. (1999) Arf proteins bind to mitotic kinesin-like protein 1 (MKLP1) in a GTP-dependent fashion. Cell Motil. Cytoskel., 44, 119–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

