Osmotic stress response in Dictyostelium is mediated by cAMP
- PMID: 11060029
- PMCID: PMC305785
- DOI: 10.1093/emboj/19.21.5782
Osmotic stress response in Dictyostelium is mediated by cAMP
Abstract
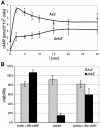
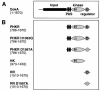
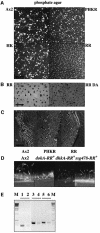
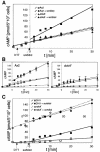
DokA, a homolog of bacterial hybrid histidine kinases, is essential for hyperosmotic stress resistance in Dictyostelium: We show that a transient intracellular cAMP signal, dependent on the presence of DokA, is generated in response to an osmotic shock. This variation of cAMP levels contributes to survival under hypertonic conditions. In contrast to the low cAMP levels observed in dokA(-) strains, overexpression of the receiver domain of DokA causes an increase in cAMP levels, resulting in a rapidly developing phenotype. We present biochemical and cell biological data indicating that the DokA receiver domain is a dominant-negative regulator of a phosphorelay, which controls the intracellular cAMP phosphodiesterase RegA. The activity of the DokA receiver domain depends on a conserved aspartate, mutation of which reverses the developmental phenotype, as well as the deregulation of cAMP metabolism.
Figures



Similar articles
-
The Evolution of Aggregative Multicellularity and Cell-Cell Communication in the Dictyostelia.J Mol Biol. 2015 Nov 20;427(23):3722-33. doi: 10.1016/j.jmb.2015.08.008. Epub 2015 Aug 15. J Mol Biol. 2015. PMID: 26284972 Free PMC article. Review.
-
An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium.EMBO J. 1998 May 15;17(10):2838-45. doi: 10.1093/emboj/17.10.2838. EMBO J. 1998. PMID: 9582277 Free PMC article.
-
Loss of the histidine kinase DhkD results in mobile mounds during development of Dictyostelium discoideum.PLoS One. 2013 Sep 25;8(9):e75618. doi: 10.1371/journal.pone.0075618. eCollection 2013. PLoS One. 2013. PMID: 24086589 Free PMC article.
-
Periodic signaling controlled by an oscillatory circuit that includes protein kinases ERK2 and PKA.Science. 2004 May 7;304(5672):875-8. doi: 10.1126/science.1094647. Science. 2004. PMID: 15131307
-
cAMP signaling in Dictyostelium. Complexity of cAMP synthesis, degradation and detection.J Muscle Res Cell Motil. 2002;23(7-8):793-802. doi: 10.1023/a:1024483829878. J Muscle Res Cell Motil. 2002. PMID: 12952077 Review.
Cited by
-
Dictyostelium stress-activated protein kinase alpha, a novel stress-activated mitogen-activated protein kinase kinase kinase-like kinase, is important for the proper regulation of the cytoskeleton.Mol Biol Cell. 2003 Nov;14(11):4526-40. doi: 10.1091/mbc.e03-01-0039. Mol Biol Cell. 2003. PMID: 14593072 Free PMC article.
-
Evolution of multicellularity in Dictyostelia.Int J Dev Biol. 2019;63(8-9-10):359-369. doi: 10.1387/ijdb.190108ps. Int J Dev Biol. 2019. PMID: 31840775 Free PMC article. Review.
-
Osmotic stress-dependent serine phosphorylation of the histidine kinase homologue DokA.BMC Biochem. 2001;2:2. doi: 10.1186/1471-2091-2-2. Epub 2001 Mar 16. BMC Biochem. 2001. PMID: 11299049 Free PMC article.
-
The Evolution of Aggregative Multicellularity and Cell-Cell Communication in the Dictyostelia.J Mol Biol. 2015 Nov 20;427(23):3722-33. doi: 10.1016/j.jmb.2015.08.008. Epub 2015 Aug 15. J Mol Biol. 2015. PMID: 26284972 Free PMC article. Review.
-
The Ca2+/calcineurin-regulated cup gene family in Dictyostelium discoideum and its possible involvement in development.Eukaryot Cell. 2004 Feb;3(1):61-71. doi: 10.1128/EC.3.1.61-71.2004. Eukaryot Cell. 2004. PMID: 14871937 Free PMC article.
References
-
- Abe K. and Yanagisawa,K. (1983) A new class of rapidly developing mutants in Dictyostelium discoideum: implications for cyclic AMP metabolism and cell differentiation. Dev. Biol., 95, 200–210. - PubMed
-
- Anjard C., Pinaud,S., Kay,R.R. and Reymond,C.D. (1992) Over expression of Dd Pk2 protein kinase causes rapid development and affects the intracellular cAMP pathway of Dictyostelium discoideum. Development, 115, 785–790. - PubMed
-
- Anjard C., Zeng,C., Loomis,W.F. and Nellen,W. (1998) Signal transduction pathways leading to spore differentiation in Dictyostelium discoideum. Dev. Biol., 193, 146–155. - PubMed
-
- Appleby J.L., Parkinson,J.S. and Bourret,R.B. (1996) Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell, 86, 845–848. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

