Quality of human immunodeficiency virus viral load testing in Australia
- PMID: 11060062
- PMCID: PMC87535
- DOI: 10.1128/JCM.38.11.4015-4020.2000
Quality of human immunodeficiency virus viral load testing in Australia
Abstract
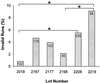
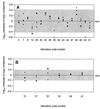
This study determined the proficiencies of laboratories measuring human immunodeficiency virus type 1 (HIV-1) viral loads and the accuracies of two assays used for HIV-1 viral load measurement in Australia and investigated the variability of the new versions of these assays. Quality assessment program panels containing (i) dilutions of HIV-1 subtype B, (ii) replicates of identical samples of HIV-1 subtype B, and (iii) samples of subtype E and B were tested by laboratories. Total variability (within and between laboratories) was tested with quality control samples. The coefficients of variation (CVs) for the Roche AMPLICOR HIV-1 MONITOR version (v) 1.0 and Chiron Quantiplex bDNA 2.0 assays ranged from 53 to 87% and 22 to 31%, respectively. The widespread occurrence of invalid runs with the AMPLICOR HIV-1 MONITOR 1.0 assay was identified. The CVs of the new versions of the assays were 82 to 86% for the AMPLICOR HIV-1 MONITOR v 1.5 assay and 16 to 23% for the Quantiplex bDNA 3.0 assay. For virus dilution samples, all but 5 of 19 laboratories obtained results within 2 standard deviations of the mean. The Quantiplex bDNA 2.0 assay reported values lower than those reported by the AMPLICOR HIV-1 MONITOR version 1.0 assay for samples containing HIV-1 subtype B, whereas the reverse was true for subtype E. Identification and resolution of the problem of invalid runs markedly improved the quality of HIV-1 viral load testing. The variability observed between laboratories and between assays, even the most recent versions, dictates that monitoring of viral load in an individual should always be by the same laboratory and by the same assay. Results for an individual which differ by less than 0.5 log(10) HIV-1 RNA copy number/ml should not be considered clinically significant.
Figures

Similar articles
-
Quantitative and cost comparison of ultrasensitive human immunodeficiency virus type 1 RNA viral load assays: Bayer bDNA quantiplex versions 3.0 and 2.0 and Roche PCR Amplicor monitor version 1.5.J Clin Microbiol. 2000 Mar;38(3):1113-20. doi: 10.1128/JCM.38.3.1113-1120.2000. J Clin Microbiol. 2000. PMID: 10699005 Free PMC article.
-
Multicenter comparison of Roche COBAS AMPLICOR MONITOR version 1.5, Organon Teknika NucliSens QT with Extractor, and Bayer Quantiplex version 3.0 for quantification of human immunodeficiency virus type 1 RNA in plasma.J Clin Microbiol. 2000 Nov;38(11):4034-41. doi: 10.1128/JCM.38.11.4034-4041.2000. J Clin Microbiol. 2000. PMID: 11060065 Free PMC article.
-
Performance characteristics of the QUANTIPLEX HIV-1 RNA 3.0 assay for detection and quantitation of human immunodeficiency virus type 1 RNA in plasma.J Clin Microbiol. 2000 Aug;38(8):2837-45. doi: 10.1128/JCM.38.8.2837-2845.2000. J Clin Microbiol. 2000. PMID: 10921936 Free PMC article.
-
The significance of HIV viral load assay precision: a review of the package insert specifications of two commercial kits.J Int Assoc Physicians AIDS Care (Chic). 2002 Oct-Dec;1(4):134-40. doi: 10.1177/154510970200100405. J Int Assoc Physicians AIDS Care (Chic). 2002. PMID: 12942672 Review.
-
Viral load: We need a new look at an old problem?J Med Virol. 2023 Aug;95(8):e29061. doi: 10.1002/jmv.29061. J Med Virol. 2023. PMID: 37638475 Review.
Cited by
-
Standardization of NAT for Blood-Borne Pathogens.Transfus Med Hemother. 2015 Jul;42(4):211-8. doi: 10.1159/000435872. Epub 2015 Jul 1. Transfus Med Hemother. 2015. PMID: 26557812 Free PMC article. Review.
-
Simultaneous Treatment of Missing Data and Measurement Error in HIV Research Using Multiple Overimputation.Epidemiology. 2015 Sep;26(5):628-36. doi: 10.1097/EDE.0000000000000334. Epidemiology. 2015. PMID: 26214336 Free PMC article.
-
International standards and reference materials for quantitative molecular infectious disease testing.J Mol Diagn. 2010 Mar;12(2):133-43. doi: 10.2353/jmoldx.2010.090067. Epub 2010 Jan 14. J Mol Diagn. 2010. PMID: 20075208 Free PMC article. Review.
-
Stability of hepatitis C virus, HIV, and hepatitis B virus nucleic acids in plasma samples after long-term storage at -20°C and -70°C.J Clin Microbiol. 2011 Sep;49(9):3163-7. doi: 10.1128/JCM.02447-10. Epub 2011 Jul 13. J Clin Microbiol. 2011. PMID: 21752974 Free PMC article.
References
-
- Berger A, Braner J, Doerr H W, Weber B. Quantification of viral load: clinical relevance for human immunodeficiency virus, hepatitis B virus and hepatitis C virus infection. Intervirology. 1998;41:24–34. - PubMed
-
- Brown A E, McNeil J G. HIV vaccine development: a subtype E-specific strategy. Southeast Asian J Trop Med Public Health. 1998;29:377–382. - PubMed
-
- Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1998. JAMA. 1998;280:78–86. - PubMed
-
- Dunne A L, Crowe S M. Comparison of branched DNA and reverse transcriptase polymerase chain reaction for quantifying six different HIV-1 subtypes in plasma. AIDS. 1997;11:126–127. - PubMed
-
- Henrard D R, Daar E, Farzadegan H, Clark S J, Phillips J, Shaw G M, Busch M P. Virologic and immunologic characterization of symptomatic and asymptomatic primary HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:305–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

