Enhanced L-type Ca2+ channel current density in coronary smooth muscle of exercise-trained pigs is compensated to limit myoplasmic free Ca2+ accumulation
- PMID: 11060122
- PMCID: PMC2270163
- DOI: 10.1111/j.1469-7793.2000.00435.x
Enhanced L-type Ca2+ channel current density in coronary smooth muscle of exercise-trained pigs is compensated to limit myoplasmic free Ca2+ accumulation
Abstract
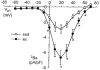
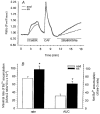
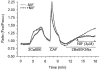
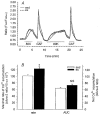
We hypothesized that enhanced voltage-gated Ca2+ channel current (VGCC) density in coronary smooth muscle cells of exercise-trained miniature Yucatan pigs is compensated by other cellular Ca2+ regulatory mechanisms to limit net myoplasmic free Ca2+ accumulation. Whole-cell voltage clamp experiments demonstrated enhanced VGCC density in smooth muscle cells freshly dispersed from coronary arteries of exercise-trained vs. sedentary animals. In separate experiments using fura-2 microfluorometry, we measured depolarization-induced (80 mM KCl) accumulation of myoplasmic free Ba2+ and free Ca2+. Both maximal rate and net accumulation of free Ba2+ in response to membrane depolarization were increased in smooth muscle cells isolated from exercise-trained pigs, consistent with an increased VGCC density. Depolarization also produced an enhanced maximal rate of free Ca2+ accumulation in cells of exercise-trained pigs; however, net accumulation of free Ca2+ was not significantly increased suggesting enhanced Ca2+ influx was compensated to limit net free Ca2+ accumulation. Inhibition of sarco-endoplasmic reticulum Ca2+-transporting ATPase (SERCA; 10 microM cyclopiazonic acid) and/or sarcolemmal Na+-Ca2+ exchange (low extracellular Na+) suggested neither mechanism compensated the enhanced VGCC in cells of exercise-trained animals. Local Ca2+-dependent inactivation of VGCC, assessed by buffering myoplasmic Ca2+ with EGTA in the pipette and using Ca2+ and Ba2+ as charge carriers, was not different between cells of sedentary and exercise-trained animals. Our findings indicate that increased VGCC density is compensated by other cellular Ca2+ regulatory mechanisms to limit net myoplasmic free Ca2+ accumulation in smooth muscle cells of exercise-trained animals. Further, SERCA, Na+-Ca2+ exchange and local Ca2+-dependent inactivation of VGCC do not appear to function as compensatory mechanisms. Additional potential compensatory mechanisms include Ca2+ extrusion via plasma membrane Ca2+-ATPase, mitochondrial uptake, myoplasmic Ca2+-binding proteins and other sources of VGCC inactivation.
Figures


References
-
- Benham CD, Tsien RW. Calcium-permeable channels in vascular smooth muscle: voltage-activated, receptor-operated, and leak channels. In: Mandel LJ, Eaton DC, editors. Cell Calcium and the Control of Membrane Transport. New York: Rockefeller University Press; 1987. pp. 49–78. - PubMed
-
- Bers DM, Bridge JHB. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum calcium pump: ryanodine and voltage sensitivity. Circulation Research. 1989;65:334–342. - PubMed
-
- Bove AA, Dewey JD. Proximal coronary vasomotor reactivity after exercise training in dogs. Circulation. 1985;71:620–625. - PubMed
-
- Bowles DK, Laughlin MH, Sturek M. Exercise training alters the Ca2+ and contractile responses of coronary arteries to endothelin. Journal of Applied Physiology. 1995;78:1079–1087. - PubMed
-
- Bowles DK, Hu Q, Laughlin MH, Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. American Journal of Physiology. 1998;275:H2159–2169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

