Patch-clamp characterisation of somatostatin-secreting -cells in intact mouse pancreatic islets
- PMID: 11060127
- PMCID: PMC2270149
- DOI: 10.1111/j.1469-7793.2000.00497.x
Patch-clamp characterisation of somatostatin-secreting -cells in intact mouse pancreatic islets
Abstract
The perforated patch whole-cell configuration of the patch-clamp technique was applied to superficial cells in intact mouse pancreatic islets. Three types of electrical activity were observed corresponding to alpha-, beta- and delta-cells. The delta-cells were electrically active in the presence of glucose but lacked the oscillatory pattern seen in the beta-cells. By contrast, the alpha-cells were electrically silent at high glucose concentrations but action potentials could be elicited by removal of the sugar. Both alpha- and beta-cells contained transient voltage-activated K+ currents. In the delta-cells, the K+ currents activated above -20 mV and were completely blocked by TEA (20 mM). The alpha-cells differed from the delta-cells in possessing a TEA-resistant K+ current activating already at -40 mV. Immunocytochemistry revealed the presence of Kv3.4 channels in delta-cells and TEA-resistant Kv4.3 channels in alpha-cells. Thus the presence of a transient TEA-resistant current can be used to functionally separate the delta- and alpha-cells. A TTX-sensitive Na+ current developed in delta-cells during depolarisations beyond -30 mV and reached a peak amplitude of 350 pA. Steady-state inactivation of this current was half-maximal at -28 mV. The delta-cells were also equipped with a sustained Ca2+ current that activated above -30 mV and reached a peak of 60 pA when measured at 2.6 mM extracellular Ca2+. A tolbutamide-sensitive KATP channel conductance was observed in delta-cells exposed to glucose-free medium. Addition of tolbutamide (0.1 mM) depolarised the delta-cell and evoked electrical activity. We propose that the KATP channels in delta-cells serve the same function as in the beta-cell and couple an elevation of the blood glucose concentration to stimulation of hormone release.
Figures



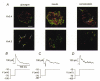
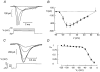

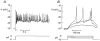

Comment in
-
The Yin and Yang of the K(ATP) channel.J Physiol. 2000 Nov 1;528(Pt 3):405. doi: 10.1111/j.1469-7793.2000.t01-1-00405.x. J Physiol. 2000. PMID: 11060118 Free PMC article. No abstract available.
References
-
- Ashcroft FM, Rorsman P. Electrophysiology of pancreatic islet cells. In: Hescheler J, Scherübl H, editors. The Electrophysiology of Neuroendocrine Cells. Boca Raton, FL, USA: CRC Press; 1995. pp. 207–243.
-
- Berts A, Ball A, Dryselius S, Gylfe E, Hellman B. Glucose stimulation of somatostatin-secreting islet cells involves oscillatory Ca2+-signalling. Endocrinology. 1996;137:693–697. - PubMed
-
- Bishop AE, Polak JM. The anatomy, organisation and ultrastructure of the islets of Langerhans. In: Pickup J, Williams G, editors. Textbook of Diabetes. Vol. 1. Oxford, UK: Blackwell Science; 1997. pp. 6.1–6.16.
-
- Conley EC. VLG K A-T. In: Conley EC, Brammar WJ, editors. The Ion Channel Facts Book: Voltage-Gated Channels. Vol. 4. San Diego: Academic Press; 1999a. pp. 196–225.
-
- Conley EC. VLG K Kv3-Shaw. In: Conley EC, Brammar WJ, editors. The Ion Channel Facts Book: Voltage-Gated Channels. Vol. 4. San Diego: Academic Press; 1999b. pp. 559–616.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

