Membrane currents in cultured human intestinal smooth muscle cells
- PMID: 11060129
- PMCID: PMC2270148
- DOI: 10.1111/j.1469-7793.2000.00521.x
Membrane currents in cultured human intestinal smooth muscle cells
Abstract
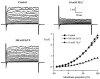
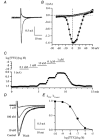
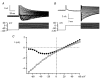
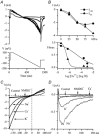
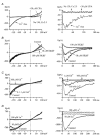
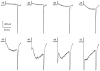
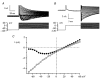
Using whole-cell patch-clamp recording techniques, we have examined voltage-gated ion currents in a cultured human intestinal smooth muscle cell line (HISM). Experiments were performed at room temperature on cells after passages 16 and 17. Two major components of the whole-cell current were a tetraethylammonium-sensitive (IC50 = 9 mM), iberiotoxin-resistant, delayed rectifier K+ current and a Na+ current inhibited by tetrodotoxin (IC50 A 100 nM). No measurable inward current via voltage-gated Ca2+ channels could be detected in these cells even with 10 mM Ca2+ or Ba2+ in the external solution. No current attributable to calcium-activated K+ channels was found and no cationic current in response to muscarinic receptor activation was present. In divalent cation-free external solution two additional currents were activated: an inwardly rectifying hyperpolarization-activated current, I(HA), and a depolarization-activated current, I(DA) x I(HA) and I(DA) could be carried by several monovalent cations; the sizes of currents in descending order were: K+ > Cs+ > Na+ for I(HA) and Na+ > K+ >> Cs+ for I(DA). I(HA) was activated and deactivated instantaneously and showed no inactivation whereas I(DA) was activated, inactivated and deactivated within tens of milliseconds. These currents were inhibited by external calcium with an IC50 of 0.3 microM for I(DA) and an IC50 of 20 microM for I(HA). Cyclopiazonic acid (CPA) induced an outward, but not an inward current. SK&F 96365, a blocker of store-operated Ca2+ channels, suppressed I(DA) with a half-maximal inhibitory concentration of 9 microM but was ineffective in inhibiting I(HA) at concentrations up to 100 microM. Gd3+ and La3+ strongly suppressed I(DA) at 1 and 10 microM, respectively and were less effective in blocking I(HA) (complete inhibition required a concentration of 100 microM for both). Carbachol at 10-100 microM evoked about a 3-fold increase in I(HA) amplitude and completely abolished I(DA). We conclude that I(HA) and I(DA) are Ca2+-blockable cationic currents with different ion selectivity profiles that are carried by different channels. I(DA) shows novel voltage-dependent properties for a cationic current.
Figures




Similar articles
-
Membrane currents recorded from a fragment of rabbit intestinal smooth muscle cell.Am J Physiol. 1986 Sep;251(3 Pt 1):C335-46. doi: 10.1152/ajpcell.1986.251.3.C335. Am J Physiol. 1986. PMID: 2428252
-
Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes.J Physiol. 1985 Jan;358:255-84. doi: 10.1113/jphysiol.1985.sp015550. J Physiol. 1985. PMID: 2580082 Free PMC article.
-
Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle.Br J Pharmacol. 1996 Jan;117(1):119-29. doi: 10.1111/j.1476-5381.1996.tb15163.x. Br J Pharmacol. 1996. PMID: 8825352 Free PMC article.
-
Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina.J Physiol. 1987 Apr;385:361-91. doi: 10.1113/jphysiol.1987.sp016497. J Physiol. 1987. PMID: 2443669 Free PMC article. Review.
-
Nonselective cation channels activated by the stimulation of muscarinic receptors in mammalian gastric smooth muscle.J Smooth Muscle Res. 2003 Dec;39(6):231-47. doi: 10.1540/jsmr.39.231. J Smooth Muscle Res. 2003. PMID: 15048016 Review.
Cited by
-
Muscarinic receptor-activated cationic channels in murine ileal myocytes.Br J Pharmacol. 2006 Sep;149(2):179-87. doi: 10.1038/sj.bjp.0706852. Epub 2006 Aug 7. Br J Pharmacol. 2006. PMID: 16894345 Free PMC article.
References
-
- Benham CD, Bolton TB, Lang RJ. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–347. - PubMed
-
- Bielefeldt K, Waite L, Abboud FM, Conklin JL. Nongenomic effects of progesterone on human intestinal smooth muscle cells. American Journal of Physiology. 1996;271:G370–376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

