Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow
- PMID: 11060135
- PMCID: PMC2270156
- DOI: 10.1111/j.1469-7793.2000.00619.x
Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow
Abstract
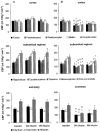
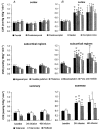
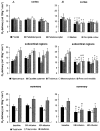
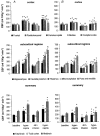
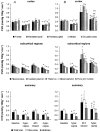
Glucocorticoid administration to women at risk of preterm delivery to accelerate fetal lung maturation has become standard practice. Antenatal glucocorticoids decrease the incidence of intraventricular haemorrhage as well as accelerating fetal lung maturation. Little is known regarding side effects on fetal cerebral function. Cortisol and synthetic glucocorticoids such as betamethasone increase fetal blood pressure and femoral vascular resistance in sheep. We determined the effects of antenatal glucocorticoid administration on cerebral blood flow (CBF) in fetal sheep. Vehicle (n = 8) or betamethasone (n = 8) was infused over 48 h via the jugular vein of chronically instrumented fetal sheep at 128 days gestation (term 146 days). The betamethasone infusion rate was that previously shown to produce fetal plasma betamethasone concentrations similar to human umbilical vein concentrations during antenatal glucocorticoid therapy. Regional CBF was measured in 10 brain regions, using coloured microspheres, before and 24 and 48 h after onset of treatment, and during hypercapnic challenges performed before and 48 h after onset of betamethasone exposure. Betamethasone exposure decreased CBF in all brain regions measured except the hippocampus after 24 h of infusion (P < 0.05). The CBF decrease was most pronounced in the thalamus and hindbrain (45-50% decrease) and least pronounced in the cortical regions (35-40% decrease). It was mediated by an increase in cerebral vascular resistance (CVR, P < 0.05) and led to a decrease in oxygen delivery to subcortical and hindbrain structures of 30-40%, to 8.6 +/- 1.1 ml x (100 g)(-1) x min(-1), and 40-45 %, to 11.0 +/- 1.6 ml x 100 g(-1) x min(-1), respectively (P < 0.05). After 48 h of betamethasone treatment, the reduction in CBF was diminished to about 25-30 %, but was still significant in comparison to vehicle-treated fetuses in all brain regions except three of the five measured cortical regions (P < 0.05). CVR and oxygen delivery were unchanged in comparison to values at 24 h of treatment. The CBF increase in response to hypercapnia was diminished (P < 0.05). These observations demonstrate for the first time that glucocorticoids exert major vasoconstrictor effects on fetal CBF. This mechanism may protect the fetus against intraventricular haemorrhage both at rest and when the fetus is challenged. Betamethasone exposure decreased the hypercapnia-induced increase in CBF (P < 0.05) due to decreased cerebral vasodilatation (P < 0.05).
Figures



References
-
- Abrams RA, Hutchison AA, Jay TM, Sokoloff L, Kennedy C. Local cerebral glucose utilization non-selectively elevated in rapid eye movement sleep of the fetus. Brain Research. 1988;468:65–70. - PubMed
-
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. - PubMed
-
- Ahima RS, Krozowski Z, Harlan RE. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. Journal of Comperative Neurology. 1991;313:522–538. - PubMed
-
- Anwar MA, Schwab M, Poston L, Nathanielsz PW. Betamethasone mediated vascular dysfunction and changes in hematological profile in the ovine fetus. American Journal of Physiology. 1999;276:H1137–1143. - PubMed
-
- Ashwal S, Dale PS, Longo LD. Regional cerebral blood flow: studies in the fetal lamb during hypoxia, hypercapnia, acidosis, and hypotension. Pediatric Research. 1984;18:1309–1316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

