Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans
- PMID: 11060136
- PMCID: PMC2270144
- DOI: 10.1111/j.1469-7793.2000.00633.x
Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans
Abstract
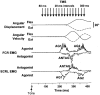
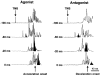
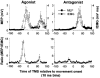
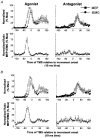
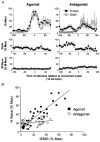
Nine healthy subjects performed single rapid wrist movements from neutral to targets at 20 deg of flexion or extension in response to an auditory cue. Surface EMG was recorded from the wrist flexors and extensors together with wrist position. Movements in both directions were characterised by the usual triphasic pattern of EMG activity in agonist (AG1), antagonist (ANTAG) and again in agonist (AG2) muscles. Single pulses of transcranial magnetic stimulation (TMS) were applied over the motor cortex at an intensity of 80 % of resting threshold at random times between 80 and 380 ms after the cue. We measured the peak-to-peak amplitude of the evoked motor potential (MEP) and the integrated EMG (IEMG) activity that preceded the MEP. In a separate set of experiments H reflexes were elicited in the wrist flexors instead of MEPs. MEP amplitudes in the agonist muscle increased by an average of 10 +/- 8 ms (range -1 to 23 ms) prior to the onset of the AG1 burst and were associated with an increase of over sevenfold in the MEP:IEMG ratio, irrespective of movement direction. Agonist H reflex amplitudes were linearly related to, and increased at the same time as, changes in agonist IEMG. The principal ANTAG burst was not preceded by an increase in the antagonist muscle MEP:IEMG ratio. No relationship was found between the amplitude of the antagonist H reflexes and the preceding antagonist IEMG. Five subjects showed an increase in the MEP:IEMG ratio preceding and during the initial part of the AG2 burst. Our method of analysis shows that changes in motor cortical excitability mediating the initiation of movement occur much closer to the onset of EMG activity (less than 23 ms) than the 80-100 ms lead time previously reported. The lack of such changes before the onset of the ANTAG burst suggests that this may be initiated by a different, perhaps subcortical, mechanism.
Figures

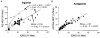
References
-
- Baker SNB, Olivier E, Lemon RN. Recording the pyramidal volley evoked by transcranial magnetic stimulation in a conscious monkey. Experimental Brain Research. 1994;99:529–533. - PubMed
-
- Berardelli A, Hallett M, Rothwell JC, Agostino R, Manfredi M, Thompson PD, Marsden CD. Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain. 1996;119:661–674. - PubMed
-
- Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Annals of Neurology. 1998;44:317–325. - PubMed
-
- Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. Journal of Neurophysiology. 1980;44:773–791. - PubMed
-
- Cheney PD, Fetz EE, Palmer SS. Patterns of facilitation and suppression of antagonist forelimb muscles from motor cortex sites in the awake monkey. Journal of Neurophysiology. 1985;53:805–820. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

