Autophagic tubes: vacuolar invaginations involved in lateral membrane sorting and inverse vesicle budding
- PMID: 11062254
- PMCID: PMC2185586
- DOI: 10.1083/jcb.151.3.519
Autophagic tubes: vacuolar invaginations involved in lateral membrane sorting and inverse vesicle budding
Abstract
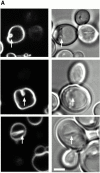
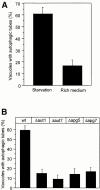
Many intracellular compartments of eukaryotic cells do not adopt a spherical shape, which would be expected in the absence of mechanisms organizing their structure. However, little is known about the principles determining the shape of organelles. We have observed very defined structural changes of vacuoles, the lysosome equivalents of yeast. The vacuolar membrane can form a large tubular invagination from which vesicles bud off into the lumen of the organelle. Formation of the tube is regulated via the Apg/Aut pathway. Its lumen is continuous with the cytosol, making this inverse budding reaction equivalent to microautophagocytosis. The tube is highly dynamic, often branched, and defined by a sharp kink of the vacuolar membrane at the site of invagination. The tube is formed by vacuoles in an autonomous fashion. It persists after vacuole isolation and, therefore, is independent of surrounding cytoskeleton. There is a striking lateral heterogeneity along the tube, with a high density of transmembrane particles at the base and a smooth zone devoid of transmembrane particles at the tip where budding occurs. We postulate a lateral sorting mechanism along the tube that mediates a depletion of large transmembrane proteins at the tip and results in the inverse budding of lipid-rich vesicles into the lumen of the organelle.
Figures







References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

