Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain
- PMID: 11062261
- PMCID: PMC2185588
- DOI: 10.1083/jcb.151.3.601
Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain
Abstract
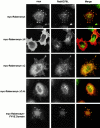
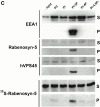
Rab5 regulates endocytic membrane traffic by specifically recruiting cytosolic effector proteins to their site of action on early endosomal membranes. We have characterized a new Rab5 effector complex involved in endosomal fusion events. This complex includes a novel protein, Rabenosyn-5, which, like the previously characterized Rab5 effector early endosome antigen 1 (EEA1), contains an FYVE finger domain and is recruited in a phosphatidylinositol-3-kinase-dependent fashion to early endosomes. Rabenosyn-5 is complexed to the Sec1-like protein hVPS45. hVPS45 does not interact directly with Rab5, therefore Rabenosyn-5 serves as a molecular link between hVPS45 and the Rab5 GTPase. This property suggests that Rabenosyn-5 is a closer mammalian functional homologue of yeast Vac1p than EEA1. Furthermore, although both EEA1 and Rabenosyn-5 are required for early endosomal fusion, only overexpression of Rabenosyn-5 inhibits cathepsin D processing, suggesting that the two proteins play distinct roles in endosomal trafficking. We propose that Rab5-dependent formation of membrane domains enriched in phosphatidylinositol-3-phosphate has evolved as a mechanism for the recruitment of multiple effector proteins to mammalian early endosomes, and that these domains are multifunctional, depending on the differing activities of the effector proteins recruited.
Figures



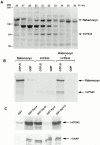



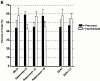
Similar articles
-
Two distinct effectors of the small GTPase Rab5 cooperate in endocytic membrane fusion.EMBO J. 1998 Apr 1;17(7):1930-40. doi: 10.1093/emboj/17.7.1930. EMBO J. 1998. PMID: 9524116 Free PMC article.
-
Sequential roles for phosphatidylinositol 3-phosphate and Rab5 in tethering and fusion of early endosomes via their interaction with EEA1.J Biol Chem. 2002 Mar 8;277(10):8611-7. doi: 10.1074/jbc.M109239200. Epub 2001 Oct 15. J Biol Chem. 2002. PMID: 11602609
-
The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding. Critical role of this dual interaction for endosomal localization.J Biol Chem. 2000 Feb 4;275(5):3699-705. doi: 10.1074/jbc.275.5.3699. J Biol Chem. 2000. PMID: 10652369
-
[Effectors of GTPase Rab5 in endocytosis and signal transduction].Postepy Biochem. 2009;55(2):171-80. Postepy Biochem. 2009. PMID: 19824473 Review. Polish.
-
Evolutionarily conserved structural and functional roles of the FYVE domain.Biochem Soc Symp. 2007;(74):95-105. doi: 10.1042/BSS0740095. Biochem Soc Symp. 2007. PMID: 17233583 Review.
Cited by
-
EHDs meet the retromer: Complex regulation of retrograde transport.Cell Logist. 2012 Jul 1;2(3):161-165. doi: 10.4161/cl.20582. Cell Logist. 2012. PMID: 23181199 Free PMC article.
-
Ultrastructural and dynamic studies of the endosomal compartment in Down syndrome.Acta Neuropathol Commun. 2020 Jun 24;8(1):89. doi: 10.1186/s40478-020-00956-z. Acta Neuropathol Commun. 2020. PMID: 32580751 Free PMC article.
-
Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity.Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2701-6. doi: 10.1073/pnas.0511138103. Epub 2006 Feb 9. Proc Natl Acad Sci U S A. 2006. PMID: 16469845 Free PMC article.
-
Systems survey of endocytosis by multiparametric image analysis.Nature. 2010 Mar 11;464(7286):243-9. doi: 10.1038/nature08779. Epub 2010 Feb 28. Nature. 2010. PMID: 20190736
-
Over-expression of Rififylin, a new RING finger and FYVE-like domain-containing protein, inhibits recycling from the endocytic recycling compartment.Mol Biol Cell. 2004 Oct;15(10):4444-56. doi: 10.1091/mbc.e04-04-0274. Epub 2004 Jun 30. Mol Biol Cell. 2004. PMID: 15229288 Free PMC article.
References
-
- Christoforidis S., Zerial M. Purification and identification of novel Rab effectors using affinity chromatography. Methods. 2000;20:403–410. - PubMed
-
- Christoforidis S., McBride H.M., Burgoyne R.D., Zerial M. The Rab5 effector EEA1 is a core component of endosome docking Nature 397 1999. 621 625a - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

