The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments
- PMID: 11062267
- PMCID: PMC2185598
- DOI: 10.1083/jcb.151.3.673
The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments
Abstract
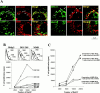
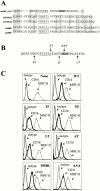
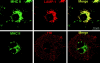
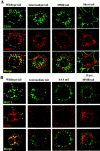
Many receptors for endocytosis recycle into and out of cells through early endosomes. We now find in dendritic cells that the DEC-205 multilectin receptor targets late endosomes or lysosomes rich in major histocompatibility complex class II (MHC II) products, whereas the homologous macrophage mannose receptor (MMR), as expected, is found in more peripheral endosomes. To analyze this finding, the cytosolic tails of DEC-205 and MMR were fused to the external domain of the CD16 Fcgamma receptor and studied in stable L cell transfectants. The two cytosolic domains each mediated rapid uptake of human immunoglobulin (Ig)G followed by recycling of intact CD16 to the cell surface. However, the DEC-205 tail recycled the CD16 through MHC II-positive late endosomal/lysosomal vacuoles and also mediated a 100-fold increase in antigen presentation. The mechanism of late endosomal targeting, which occurred in the absence of human IgG, involved two functional regions: a membrane-proximal region with a coated pit sequence for uptake, and a distal region with an EDE triad for the unusual deeper targeting. Therefore, the DEC-205 cytosolic domain mediates a new pathway of receptor-mediated endocytosis that entails efficient recycling through late endosomes and a greatly enhanced efficiency of antigen presentation to CD4(+) T cells.
Figures


References
-
- Amigorena S., Bonnerot C., Drake J.R., Choquet D., Hunziker W., Guillet J.-G., Webster P., Sautes C., Mellman I., Fridman W.H. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes Science. 256 1992. 1808 1812a - PubMed
-
- Amigorena S., Salamero J., Davoust J., Fridman W.H., Bonnerot C. Tyrosine-containing motif that transduces cell activation signals also determines internalization and antigen presentation via type III receptors for IgG Nature. 358 1992. 337 341b - PubMed
-
- Bonnerot C., Lankar D. Role of B cell receptor Igα and Igβ subunits in MHC class II-restricted antigen presentation. Immunity. 1995;3:335–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

