Evidence that beta3 integrin-induced Rac activation involves the calpain-dependent formation of integrin clusters that are distinct from the focal complexes and focal adhesions that form as Rac and RhoA become active
- PMID: 11062268
- PMCID: PMC2185596
- DOI: 10.1083/jcb.151.3.685
Evidence that beta3 integrin-induced Rac activation involves the calpain-dependent formation of integrin clusters that are distinct from the focal complexes and focal adhesions that form as Rac and RhoA become active
Abstract
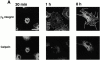
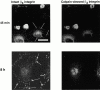
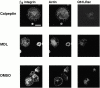
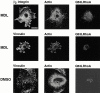
Interaction of integrins with the extracellular matrix leads to transmission of signals, cytoskeletal reorganizations, and changes in cell behavior. While many signaling molecules are known to be activated within Rac-induced focal complexes or Rho-induced focal adhesions, the way in which integrin-mediated adhesion leads to activation of Rac and Rho is not known. In the present study, we identified clusters of integrin that formed upstream of Rac activation. These clusters contained a Rac-binding protein(s) and appeared to be involved in Rac activation. The integrin clusters contained calpain and calpain-cleaved beta3 integrin, while the focal complexes and focal adhesions that formed once Rac and Rho were activated did not. Moreover, the integrin clusters were dependent on calpain for their formation. In contrast, while Rac- and Rho-GTPases were dependent on calpain for their activation, formation of focal complexes and focal adhesions by constitutively active Rac or Rho, respectively, occurred even when calpain inhibitors were present. Taken together, these data are consistent with a model in which integrin-induced Rac activation requires the formation of integrin clusters. The clusters form in a calpain-dependent manner, contain calpain, calpain-cleaved integrin, and a Rac binding protein(s). Once Rac is activated, other integrin signaling complexes are formed by a calpain-independent mechanism(s).
Figures








References
-
- Banno Y., Nakashima S., Hachiya T., Nozawa Y. Endogenous cleavage of phospholipase C-b3 by agonist-induced activation of calpain in human platelets. J. Biol. Chem. 1995;270:4318–4324. - PubMed
-
- Beckerle M.C., Burridge K., DeMartino G.N., Croall D.E. Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell. 1987;51:569–577. - PubMed
-
- Boguski M.S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. - PubMed
-
- Bokoch G.M., Der C.J. Emerging concepts in the Ras superfamily of GTP-binding proteins. FASEB J. 1993;7:750–759. - PubMed
-
- Bollag G., McCormick F. Regulators and effectors of ras proteins. Annu. Rev. Cell Biol. 1991;7:601–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

