Apolipoprotein J/clusterin limits the severity of murine autoimmune myocarditis
- PMID: 11067863
- PMCID: PMC301413
- DOI: 10.1172/JCI9037
Apolipoprotein J/clusterin limits the severity of murine autoimmune myocarditis
Abstract
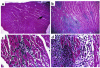
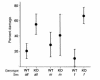
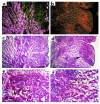
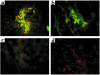
Apolipoprotein J/clusterin (apoJ/clusterin), an intriguing protein with unknown function, is induced in myocarditis and numerous other inflammatory injuries. To test its ability to modify myosin-induced autoimmune myocarditis, we generated apoJ-deficient mice. ApoJ-deficient and wild-type mice exhibited similar initial onset of myocarditis, as evidenced by the induction of two early markers of the T cell-mediated immune response, MHC-II and TNF receptor p55. Furthermore, autoantibodies against the primary antigen cardiac myosin were induced to the same extent. Although the same proportion of challenged animals exhibited some degree of inflammatory infiltrate, inflammation was more severe in apoJ-deficient animals. Inflammatory lesions were more diffuse and extensive in apoJ-deficient mice, particularly in females. In marked contrast to wild-type animals, the development of a strong generalized secondary response against cardiac antigens in apoJ-deficient mice was predictive of severe myocarditis. Wild-type mice with a strong Ab response to secondary antigens appeared to be protected from severe inflammation. After resolution of inflammation, apoJ-deficient, but not wild-type, mice exhibited cardiac function impairment and severe myocardial scarring. These results suggest that apoJ limits progression of autoimmune myocarditis and protects the heart from postinflammatory tissue destruction.
Figures




Similar articles
-
Apolipoprotein J/clusterin induction in myocarditis: A localized response gene to myocardial injury.Am J Pathol. 1996 Jun;148(6):1971-83. Am J Pathol. 1996. PMID: 8669482 Free PMC article.
-
Low-molecular-weight tumor necrosis factor receptor p55 controls induction of autoimmune heart disease.Circulation. 1997 Feb 4;95(3):655-61. doi: 10.1161/01.cir.95.3.655. Circulation. 1997. PMID: 9024154
-
Cardiac myosin-induced myocarditis: target recognition by autoreactive T cells requires prior activation of cardiac interstitial cells.Lab Invest. 1996 May;74(5):845-52. Lab Invest. 1996. PMID: 8642780
-
Cellular and molecular mechanisms of murine autoimmune myocarditis.APMIS. 1997 Jan;105(1):1-13. doi: 10.1111/j.1699-0463.1997.tb00532.x. APMIS. 1997. PMID: 9063494 Review.
-
Pathogenesis of myocarditis and dilated cardiomyopathy.Adv Immunol. 2008;99:95-114. doi: 10.1016/S0065-2776(08)00604-4. Adv Immunol. 2008. PMID: 19117533 Review.
Cited by
-
Clusterin Seals the Ocular Surface Barrier in Mouse Dry Eye.PLoS One. 2015 Sep 24;10(9):e0138958. doi: 10.1371/journal.pone.0138958. eCollection 2015. PLoS One. 2015. PMID: 26402857 Free PMC article.
-
Extracellular Amyloid Deposits in Alzheimer's and Creutzfeldt-Jakob Disease: Similar Behavior of Different Proteins?Int J Mol Sci. 2020 Dec 22;22(1):7. doi: 10.3390/ijms22010007. Int J Mol Sci. 2020. PMID: 33374972 Free PMC article. Review.
-
Regulation of CLU gene expression by oncogenes and epigenetic factors implications for tumorigenesis.Adv Cancer Res. 2009;105:115-32. doi: 10.1016/S0065-230X(09)05007-6. Adv Cancer Res. 2009. PMID: 19879426 Free PMC article. Review.
-
Interaction of clusterin and matrix metalloproteinase-9 and its implication for epithelial homeostasis and inflammation.Am J Pathol. 2012 May;180(5):2028-39. doi: 10.1016/j.ajpath.2012.01.025. Epub 2012 Mar 20. Am J Pathol. 2012. PMID: 22440257 Free PMC article.
-
Clusterin facilitates apoptotic cell clearance and prevents apoptotic cell-induced autoimmune responses.Cell Death Dis. 2016 May 5;7(5):e2215. doi: 10.1038/cddis.2016.113. Cell Death Dis. 2016. PMID: 27148688 Free PMC article.
References
-
- Friman G. The incidence and epidemiology of myocarditis. Eur Heart J. 1999;20:1063–1066. - PubMed
-
- Neumann DA, Burek CL, Baughman KL, Rose NR, Herskowitz A. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol. 1990;16:839–846. - PubMed
-
- Maisch B, et al. Immunological cellular regulator and effector mechanisms in myocarditis. Herz. 1985;10:8–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

