Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology
- PMID: 11067880
- PMCID: PMC2193356
- DOI: 10.1084/jem.192.9.1317
Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology
Abstract
The effect of infection history is ignored in most animal models of infectious disease. The attachment protein of respiratory syncytial virus (RSV) induces T helper cell type 2-driven pulmonary eosinophilia in mice similar to that seen in the failed infant vaccinations in the 1960s. We show that previous influenza virus infection of mice: (a) protects against weight loss, illness, and lung eosinophilia; (b) attenuates recruitment of inflammatory cells; and (c) reduces cytokine secretion caused by RSV attachment protein without affecting RSV clearance. This protective effect can be transferred via influenza-immune splenocytes to naive mice and is long lived. Previous immunity to lung infection clearly plays an important and underestimated role in subsequent vaccination and infection. The data have important implications for the timing of vaccinations in certain patient groups, and may contribute to variability in disease susceptibility observed in humans.
Figures


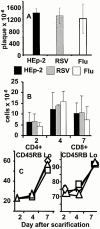



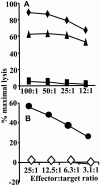
References
-
- Prescott S.L., Macaubas C., Smallacombe T., Holt B.J., Sly P.D., Holt P.G. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. - PubMed
-
- Romagnani S. Induction of TH1 and TH2 responsesa key role for the ‘natural’ immune response? Immunol. Today. 1992;13:379–381. - PubMed
-
- Martinez F.D., Wright A.L., Taussig L.M., Holberg C.J., Halonen M., Morgan W.J. Asthma and wheezing in the first six years of life. N. Engl. J. Med. 1995;332:133–138. - PubMed
-
- Holt P.G., Macaubas C. Development of long-term tolerance versus sensitization to environmental allergens during the perinatal period. Curr. Opin. Immunol. 1997;9:782–787. - PubMed
-
- Shirakawa T., Enomoto T., Shimazu S., Hopkin J.M. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

