Identification of a novel prostaglandin f(2alpha) synthase in Trypanosoma brucei
- PMID: 11067881
- PMCID: PMC2193354
- DOI: 10.1084/jem.192.9.1327
Identification of a novel prostaglandin f(2alpha) synthase in Trypanosoma brucei
Abstract
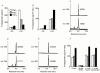
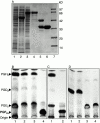
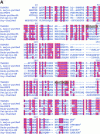
Members of the genus Trypanosoma cause African trypanosomiasis in humans and animals in Africa. Infection of mammals by African trypanosomes is characterized by an upregulation of prostaglandin (PG) production in the plasma and cerebrospinal fluid. These metabolites of arachidonic acid (AA) may, in part, be responsible for symptoms such as fever, headache, immunosuppression, deep muscle hyperaesthesia, miscarriage, ovarian dysfunction, sleepiness, and other symptoms observed in patients with chronic African trypanosomiasis. Here, we show that the protozoan parasite T. brucei is involved in PG production and that it produces PGs enzymatically from AA and its metabolite, PGH(2). Among all PGs synthesized, PGF(2alpha) was the major prostanoid produced by trypanosome lysates. We have purified a novel T. brucei PGF(2alpha) synthase (TbPGFS) and cloned its cDNA. Phylogenetic analysis and molecular properties revealed that TbPGFS is completely distinct from mammalian PGF synthases. We also found that TbPGFS mRNA expression and TbPGFS activity were high in the early logarithmic growth phase and low during the stationary phase. The characterization of TbPGFS and its gene in T. brucei provides a basis for the molecular analysis of the role of parasite-derived PGF(2alpha) in the physiology of the parasite and the pathogenesis of African trypanosomiasis.
Figures


Similar articles
-
Prostaglandin production from arachidonic acid and evidence for a 9,11-endoperoxide prostaglandin H2 reductase in Leishmania.Int J Parasitol. 2002 Dec 19;32(14):1693-700. doi: 10.1016/s0020-7519(02)00160-1. Int J Parasitol. 2002. Corrected and republished in: Int J Parasitol. 2003 Feb;33(2):221-8. doi: 10.1016/s0020-7519(02)00254-0. PMID: 12464415 Corrected and republished.
-
Prostaglandin production from arachidonic acid and evidence for a 9,11-endoperoxide prostaglandin H2 reductase in Leishmania.Int J Parasitol. 2003 Feb;33(2):221-8. doi: 10.1016/s0020-7519(02)00254-0. Int J Parasitol. 2003. PMID: 12633659
-
Crystallization and preliminary X-ray crystallographic studies of Trypanosoma brucei prostaglandin F(2 alpha) synthase.J Biochem. 2002 Dec;132(6):859-61. doi: 10.1093/oxfordjournals.jbchem.a003298. J Biochem. 2002. PMID: 12473187
-
[PGF synthetase].Seikagaku. 1986 Jan;58(1):23-8. Seikagaku. 1986. PMID: 3084680 Review. Japanese. No abstract available.
-
Molecular biology of prostanoid biosynthetic enzymes and receptors.Adv Exp Med Biol. 1997;400B:989-1011. Adv Exp Med Biol. 1997. PMID: 9547656 Review. No abstract available.
Cited by
-
Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection.J Exp Med. 2007 Apr 16;204(4):929-40. doi: 10.1084/jem.20062432. Epub 2007 Apr 9. J Exp Med. 2007. PMID: 17420269 Free PMC article.
-
A role for trypanosomatid aldo-keto reductases in methylglyoxal, prostaglandin and isoprostane metabolism.Biochem J. 2018 Aug 30;475(16):2593-2610. doi: 10.1042/BCJ20180232. Biochem J. 2018. PMID: 30045874 Free PMC article.
-
Fatty Acid Composition and Metabolism in Leishmania Parasite Species: Potential Biomarkers or Drug Targets for Leishmaniasis?Int J Mol Sci. 2023 Feb 28;24(5):4702. doi: 10.3390/ijms24054702. Int J Mol Sci. 2023. PMID: 36902138 Free PMC article. Review.
-
Trypanosoma cruzi Produces the Specialized Proresolving Mediators Resolvin D1, Resolvin D5, and Resolvin E2.Infect Immun. 2018 Mar 22;86(4):e00688-17. doi: 10.1128/IAI.00688-17. Print 2018 Apr. Infect Immun. 2018. PMID: 29358332 Free PMC article.
-
Bioactive lipids in Trypanosoma cruzi infection.Adv Parasitol. 2011;76:1-31. doi: 10.1016/B978-0-12-385895-5.00001-3. Adv Parasitol. 2011. PMID: 21884885 Free PMC article. Review.
References
-
- Smith W.L., DeWitt D.L. Prostaglandin endoperoxide H synthases-1 and -2. Adv. Immunol. 1996;62:167–215. - PubMed
-
- Samuelsson B. Prostaglandins, thromboxanes, and leukotrienesformation and biological roles. Harvey Lect. 1979;75:1–40. - PubMed
-
- Mathé A.A., Hedqvist P., Strandberg K., Leslie C.A. Aspects of prostaglandin function in the lung (second of two parts) N. Engl. J. Med. 1977;296:910–914. - PubMed
-
- Oliw E., Granström E., Änggard E. The prostaglandins and essential fatty acids. In: Pace-Asciak C., Granström E., editors. Prostaglandins and Related Substances. Elsevier; Amsterdam: 1983. pp. 11–19.
-
- Glew R.H. Prostaglandins and thromboxanes. In: Devlin T.M., editor. Textbook of Biochemistry with Clinical Correlations. Wiley-Liss, Inc.; New York: 1992. pp. 461–466.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

