The amino terminus targets the mixed lineage leukemia (MLL) protein to the nucleolus, nuclear matrix and mitotic chromosomal scaffolds
- PMID: 11069025
- PMCID: PMC7543881
- DOI: 10.1038/sj.leu.2401933
The amino terminus targets the mixed lineage leukemia (MLL) protein to the nucleolus, nuclear matrix and mitotic chromosomal scaffolds
Abstract
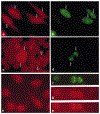
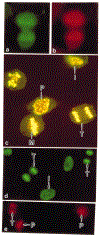
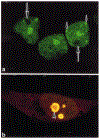
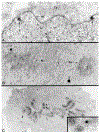
The mixed-lineage leukemia gene (MLL) is associated with more than 25 chromosomal translocations involving band 11q23 in diverse subtypes of human acute leukemia. Conditional expression of a 50 kDa amino terminal fragment spanning the AT hook motifs of MLL (MLL3AT) causes cell cycle arrest, upregulation of p21Cip1 and p27KiP1 and partial monocytic differentiation of the monoblastic U937 cell line, suggesting a major role for MLL3AT in MLL-AF9-induced myelomonocytic differentiation. In this study, we analyzed the subcellular localization of conditionally expressed MLL3AT in both U937 and HeLa cell lines. Immunofluorescence staining, confocal laser scanning microscopy and immunoelectron microscopy indicated that MLL3AT, like endogenous MLL, localized in the nucleoplasm in a punctate pattern of distribution, including regions attached to the nuclear envelope and the periphery of the nucleolus. We found that MLL3AT and endogenous MLL were present in interphase nuclear matrices and colocalized with topoisomerase II to mitotic chromosomal scaffolds. Nucleoplasm and nucleolar localization was observed even for MLL-AF9 and MLL-AF4 conditionally expressed chimeric proteins, suggesting a common target conferred by the amino terminus of MLL to many if not all the chimeric MLL proteins. The nuclear matrix/scaffold association suggests a role for the amino terminus of MLL in the modulation of chromatin structure, leading to epigenetic effects on the maintenance of gene expression.
Figures



References
-
- Ziemin-van der Poel S, McCabe NR, Gill HJ, Espinosa R III, Patel Y, Harden A, Rubinelli P, Smith SD, LeBeau Mm, Rowley JD, Diaz MO. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci USA 1991; 88: 10735–10739. - PMC - PubMed
-
- Raimondi SC, Peiper SC, Kitchingman G, Behm FG, Williams DL, Hancock ML, Mirro J Jr. Childhood acute lymphoblastic leukemia with chromosomal breakpoints at 11q23. Blood 1989; 73: 1627–1634. - PubMed
-
- Raimondi SC, Kalwinsky DK, Hayashi Y, Behm FG, Mirro J Jr, Williams DL. Cytogenetics of childhood acute nonlymphocytic leukemia. Cancer Genet Cytogenet 1989; 40: 13–27. - PubMed
-
- DeVore R, Whitlock J, Hainsworth JD, Johnson DH. Therapy-related acute nonlymphocytic leukemia with monocytic features and rearrangement of chromosome 11q. Ann Intern Med 1989; 110: 740–742. - PubMed
-
- Pui C- H, Behm FG, Raimondi SC, Dodge RK, George SL, Rivera GK, Mirro J Jr, Kalwinsky DK, Dahl GV, Murphy SB. Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. New Engl J Med 1989; 321: 136–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

