Multiple quantum solid-state NMR indicates a parallel, not antiparallel, organization of beta-sheets in Alzheimer's beta-amyloid fibrils
- PMID: 11069287
- PMCID: PMC27175
- DOI: 10.1073/pnas.230315097
Multiple quantum solid-state NMR indicates a parallel, not antiparallel, organization of beta-sheets in Alzheimer's beta-amyloid fibrils
Abstract
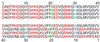
Senile plaques associated with Alzheimer's disease contain deposits of fibrils formed by 39- to 43-residue beta-amyloid peptides with possible neurotoxic effects. X-ray diffraction measurements on oriented fibril bundles have indicated an extended beta-sheet structure for Alzheimer's beta-amyloid fibrils and other amyloid fibrils, but the supramolecular organization of the beta-sheets and other structural details are not well established because of the intrinsically noncrystalline, insoluble nature of amyloid fibrils. Here we report solid-state NMR measurements, using a multiple quantum (MQ) (13)C NMR technique, that probe the beta-sheet organization in fibrils formed by the full-length, 40-residue beta-amyloid peptide (Abeta(1-40)). Although an antiparallel beta-sheet organization often is assumed and is invoked in recent structural models for full-length beta-amyloid fibrils, the MQNMR data indicate an in-register, parallel organization. This work provides site-specific, atomic-level structural constraints on full-length beta-amyloid fibrils and applies MQNMR to a significant problem in structural biology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

