Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity
- PMID: 11069288
- PMCID: PMC27231
- DOI: 10.1073/pnas.230362997
Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity
Abstract
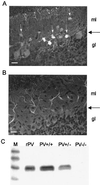
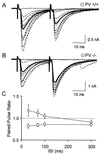
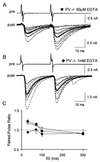
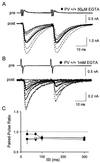
GABAergic (GABA = gamma-aminobutyric acid) neurons from different brain regions contain high levels of parvalbumin, both in their soma and in their neurites. Parvalbumin is a slow Ca(2+) buffer that may affect the amplitude and time course of intracellular Ca(2+) transients in terminals after an action potential, and hence may regulate short-term synaptic plasticity. To test this possibility, we have applied paired-pulse stimulations (with 30- to 300-ms intervals) at GABAergic synapses between interneurons and Purkinje cells, both in wild-type (PV+/+) mice and in parvalbumin knockout (PV-/-) mice. We observed paired-pulse depression in PV+/+ mice, but paired-pulse facilitation in PV-/- mice. In paired recordings of connected interneuron-Purkinje cells, dialysis of the presynaptic interneuron with the slow Ca(2+) buffer EGTA (1 mM) rescues paired-pulse depression in PV-/- mice. These data show that parvalbumin potently modulates short-term synaptic plasticity.
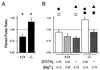
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

