Pharmacokinetics of propionyl-L-carnitine in humans: evidence for saturable tubular reabsorption
- PMID: 11069438
- PMCID: PMC2014409
- DOI: 10.1046/j.1365-2125.2000.00280.x
Pharmacokinetics of propionyl-L-carnitine in humans: evidence for saturable tubular reabsorption
Abstract
Aims: Propionyl-L-carnitine (PLC) is an endogenous compound which, along with L-carnitine (LC) and acetyl-L-carnitine (ALC), forms a component of the endogenous carnitine pool in humans and most, if not all, animal species. PLC is currently under investigation for the treatment of peripheral artery disease, and the present study was conducted to assess the pharmacokinetics of intravenous propionyl-L-carnitine hydrochloride.
Methods: This was a placebo-controlled, double-blind, parallel group, dose-escalating study in which 24 healthy males were divided into four groups of six. Four subjects from each group received propionyl-L-carnitine hydrochloride and two received placebo. The doses (1 g, 2 g, 4 g and 8 g) were administered as a constant rate infusion over 2 h and blood and urine were collected for 24 h from the start of the infusion. PLC, ALC and LC in plasma and urine were quantified by h.p. l.c.
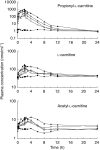
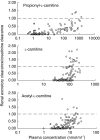
Results: All 24 subjects successfully completed the study and the infusions were well tolerated. In addition to the expected increase in PLC levels, the plasma concentrations and urinary excretion of LC and ALC also increased above baseline values following intravenous propionyl-L-carnitine hydrochloride administration. At a dose of 1 g, PLC was found to have a mean (+/- s.d.) half-life of 1.09 +/- 0.15 h, a clearance of 11.6 +/- 0.24 l h-1 and a volume of distribution of 18.3 +/- 2.4 l. None of these parameters changed with dose. In placebo-treated subjects, endogenous PLC, LC and ALC underwent extensive renal tubular reabsorption, as indicated by renal excretory clearance to GFR ratios of less than 0.1. The renal-excretory clearance of PLC, which was 0.33 +/- 0.38 l h-1 under baseline condition, increased (P < 0. 001) from 1.98 +/- 0.59 l h-1 at a dose of 1 g to 5.55 +/- 1.50 l h-1 at a dose of 8 g (95% confidence interval for the difference was 2.18,4.97). As a consequence, the percent of the dose excreted unchanged in urine increased (P < 0.001) from 18.1 +/- 5.5% (1 g) to 50.3 +/- 13.3% (8 g). The renal-excretory clearance of LC and ALC also increased substantially after PLC administration and there was evidence for renal metabolism of PLC to LC and ALC.
Conclusions: Intravenous administration of propionyl-L-carnitine hydrochloride caused significant increases in the renal excretory clearances of PLC, LC and ALC, due to saturation of the renal tubular reabsorption process - as a consequence there was a substantial increase with dose in the fraction excreted unchanged in urine. Despite the marked increase in the renal clearance of PLC, total clearance remained unchanged, suggesting a compensatory reduction in the clearance of the compound by non excretory routes.
Figures
Similar articles
-
Pharmacokinetics of L-carnitine.Clin Pharmacokinet. 2003;42(11):941-67. doi: 10.2165/00003088-200342110-00002. Clin Pharmacokinet. 2003. PMID: 12908852 Review.
-
Comparison of pharmacokinetics of L-carnitine, acetyl-L-carnitine and propionyl-L-carnitine after single oral administration of L-carnitine in healthy volunteers.Clin Invest Med. 2009 Feb 1;32(1):E13-9. doi: 10.25011/cim.v32i1.5082. Clin Invest Med. 2009. PMID: 19178874
-
Excretion and metabolism of propionyl-L-carnitine in the isolated perfused rat kidney.J Pharmacol Exp Ther. 1997 Jun;281(3):1071-6. J Pharmacol Exp Ther. 1997. PMID: 9190838
-
Urinary excretion of L-carnitine and its short-chain acetyl-L-carnitine in patients undergoing carboplatin treatment.Cancer Chemother Pharmacol. 2007 Jun;60(1):19-26. doi: 10.1007/s00280-006-0341-3. Epub 2006 Sep 20. Cancer Chemother Pharmacol. 2007. PMID: 16988826 Clinical Trial.
-
Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism.Ann N Y Acad Sci. 2004 Nov;1033:30-41. doi: 10.1196/annals.1320.003. Ann N Y Acad Sci. 2004. PMID: 15591001 Review.
Cited by
-
Metabolomics analysis for hydroxy-L-proline-induced calcium oxalate nephrolithiasis in rats based on ultra-high performance liquid chromatography quadrupole time-of-flight mass spectrometry.Sci Rep. 2016 Jul 22;6:30142. doi: 10.1038/srep30142. Sci Rep. 2016. PMID: 27443631 Free PMC article.
-
Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects.Clin Pharmacokinet. 2012 Sep 1;51(9):553-72. doi: 10.1007/BF03261931. Clin Pharmacokinet. 2012. PMID: 22804748 Review.
-
Intermittent claudication: new targets for drug development.Drugs. 2013 Jul;73(10):999-1014. doi: 10.1007/s40265-013-0078-3. Drugs. 2013. PMID: 23775528 Review.
-
Pharmacokinetics of L-carnitine.Clin Pharmacokinet. 2003;42(11):941-67. doi: 10.2165/00003088-200342110-00002. Clin Pharmacokinet. 2003. PMID: 12908852 Review.
References
-
- Brevetti G, Perna S, Sabba C, Martone VD, Di Iorio A, Barlatta G. Effect of propionyl-l-carnitine on quality of life in intermittent claudication. Am J Cardiol. 1997;79:777–780. 10.1016/s0002-9149(96)00867-3. - DOI - PubMed
-
- Brevetti G, Perna S, Sabba C, Martone VD, Condorelli M. Propionyl-l-carnitine in intermittent claudication: double-blind, placebo-controlled, dose titration, multicentre study. J Am Coll Cardiol. 1997;26:1411–1416. 10.1016/0735-1097(95)00344-4. - DOI - PubMed
-
- Wiseman LR, Brogden RN. Propionyl-l-carnitine. Drugs Aging. 1998;12:243–250. - PubMed
-
- Bahl JJ, Bressler R. The pharmacology of carnitine. Ann Rev Pharmacol Toxicol. 1987;27:257–277. - PubMed
-
- Ferrari R, De Giuli F. The propionyl-l-carnitine hypothesis: an alternative approach to treating heart failure. J Card Fail. 1997;3:217–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

