Regulation of cellular growth by the Drosophila target of rapamycin dTOR
- PMID: 11069888
- PMCID: PMC317034
- DOI: 10.1101/gad.835000
Regulation of cellular growth by the Drosophila target of rapamycin dTOR
Abstract
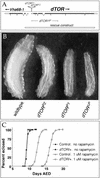
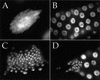
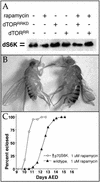
The TOR protein kinases (TOR1 and TOR2 in yeast; mTOR/FRAP/RAFT1 in mammals) promote cellular proliferation in response to nutrients and growth factors, but their role in development is poorly understood. Here, we show that the Drosophila TOR homolog dTOR is required cell autonomously for normal growth and proliferation during larval development, and for increases in cellular growth caused by activation of the phosphoinositide 3-kinase (PI3K) signaling pathway. As in mammalian cells, the kinase activity of dTOR is required for growth factor-dependent phosphorylation of p70 S6 kinase (p70(S6K)) in vitro, and we demonstrate that overexpression of p70(S6K) in vivo can rescue dTOR mutant animals to viability. Loss of dTOR also results in cellular phenotypes characteristic of amino acid deprivation, including reduced nucleolar size, lipid vesicle aggregation in the larval fat body, and a cell type-specific pattern of cell cycle arrest that can be bypassed by overexpression of the S-phase regulator cyclin E. Our results suggest that dTOR regulates growth during animal development by coupling growth factor signaling to nutrient availability.
Figures





References
-
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. - PubMed
-
- Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. - PubMed
-
- Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell. 1999;97:865–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
