Developmental expression of muscarinic acetylcholine receptors in chick retina: selective induction of M2 muscarinic receptor expression in ovo by a factor secreted by muller glial cells
- PMID: 11069949
- PMCID: PMC6773186
- DOI: 10.1523/JNEUROSCI.20-22-08417.2000
Developmental expression of muscarinic acetylcholine receptors in chick retina: selective induction of M2 muscarinic receptor expression in ovo by a factor secreted by muller glial cells
Abstract
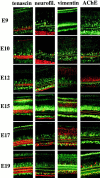
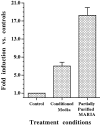
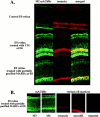
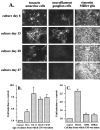
Muscarinic acetylcholine receptors (mAChRs) play an important role in signal processing in the retina. We have used subtype-specific antibodies to identify the changes in the localization of mAChR expression during embryonic development of the retina in vivo and their relationship to the changes in mAChRs in retinal cells in culture. We have demonstrated previously that treatment of fresh retinal cultures with conditioned media from mature retinal cultures specifically induces expression of the M(2) mAChR (McKinnon et al., 1998). We show that the M(2)-inducing activity, which we tentatively have called MARIA (muscarinic acetylcholine receptor-inducing activity) is produced by Müller glial cells in culture, because significant activity can be found in media conditioned by essentially neuron-free cultures of Müller glia, as well as by a Müller glial cell line but not several neuroblastoma cell lines. We also demonstrate that the appearance of the M(2) receptor in vivo occurs concomitantly with the appearance of significant numbers of Müller glial cells in the developing retina. Furthermore, the administration of crude or partially purified preparations of MARIA to developing chick embryos in ovo induces precocious expression of M(2) mAChRs in the appropriate cell types in the retina. These results show that a factor secreted by cultured retinal Müller glia can regulate M(2) mAChR expression in vivo and in vitro and suggest that the secretion of MARIA by Müller glia in vivo may be responsible for the normal induction of M(2) mAChR expression during embryonic development.
Figures


Similar articles
-
Developmental regulation of the cm2 muscarinic acetylcholine receptor gene: selective induction by a secreted factor produced by embryonic chick retinal cells.J Neurosci. 1998 Jan 1;18(1):59-69. doi: 10.1523/JNEUROSCI.18-01-00059.1998. J Neurosci. 1998. PMID: 9412486 Free PMC article.
-
Peripapillary glial cells in the chick retina: A special glial cell type expressing astrocyte, radial glia, neuron, and oligodendrocyte markers throughout development.Glia. 2004 May;46(4):346-55. doi: 10.1002/glia.10351. Glia. 2004. PMID: 15095365
-
Glutathione-Induced Calcium Shifts in Chick Retinal Glial Cells.PLoS One. 2016 Apr 14;11(4):e0153677. doi: 10.1371/journal.pone.0153677. eCollection 2016. PLoS One. 2016. PMID: 27078878 Free PMC article.
-
Molecular analysis of the regulation of muscarinic receptor expression and function.Life Sci. 1999;64(6-7):375-9. doi: 10.1016/s0024-3205(98)00577-3. Life Sci. 1999. PMID: 10069499 Review.
-
Regulation of muscarinic receptor expression and function in cultured cells and in knock-out mice.Life Sci. 1997;60(13-14):1101-4. doi: 10.1016/s0024-3205(97)00053-2. Life Sci. 1997. PMID: 9121353 Review.
Cited by
-
Müller glia as an active compartment modulating nervous activity in the vertebrate retina: neurotransmitters and trophic factors.Neurochem Res. 2008 Aug;33(8):1466-74. doi: 10.1007/s11064-008-9604-1. Epub 2008 Feb 14. Neurochem Res. 2008. PMID: 18273703 Review.
-
Fluorescent styryl dyes FM1-43 and FM2-10 are muscarinic receptor antagonists: intravital visualization of receptor occupancy.J Physiol. 2006 Aug 15;575(Pt 1):23-35. doi: 10.1113/jphysiol.2006.106351. Epub 2006 May 25. J Physiol. 2006. PMID: 16728454 Free PMC article.
-
Müller Glia in Retinal Development: From Specification to Circuit Integration.Front Neural Circuits. 2022 Feb 4;15:815923. doi: 10.3389/fncir.2021.815923. eCollection 2021. Front Neural Circuits. 2022. PMID: 35185477 Free PMC article. Review.
-
Muscarinic Acetylcholine Receptors in the Retina-Therapeutic Implications.Int J Mol Sci. 2021 May 8;22(9):4989. doi: 10.3390/ijms22094989. Int J Mol Sci. 2021. PMID: 34066677 Free PMC article. Review.
-
Neuron-glia signaling in developing retina mediated by neurotransmitter spillover.Elife. 2015 Aug 14;4:e09590. doi: 10.7554/eLife.09590. Elife. 2015. PMID: 26274565 Free PMC article.
References
-
- Bartsch S, Husmann K, Schachner M, Bartsch U. The extracellular matrix molecule tenascin: expression in the developing chick retinotectal system and substrate properties for retinal ganglion cell neurites in vitro. Eur J Neurosci. 1995;7:907–916. - PubMed
-
- Bonaventure N, Jardon B, Sahel J, Wioland N. Neurotransmission in the frog retina: possible physiological and histological correlations. Doc Ophthalmol. 1989;72:71–82. - PubMed
-
- Bradshaw AD, McNagny KM, Gervin DB, Can GM, Graf T, Clegg DO. Integrin a2b1 mediates interactions between developing embryonic retinal cells and collagen. Development. 1995;121:3593–3602. - PubMed
-
- Catsicas M, Bonness V, Becker D, Mobbs P. Spontaneous Ca+2 transients and their transmission in the developing chick retina. Curr Biol. 1997;8:283–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
