Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity
- PMID: 11069952
- PMCID: PMC6773171
- DOI: 10.1523/JNEUROSCI.20-22-08443.2000
Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity
Abstract
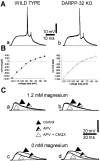
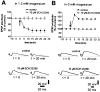
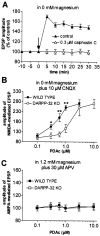
A complex chain of intracellular signaling events, critically important in motor control, is activated by the stimulation of D1-like dopamine (DA) receptors in striatal neurons. At corticostriatal synapses on medium spiny neurons, we provide evidence that the D1-like receptor-dependent activation of DA and cyclic adenosine 3',5' monophosphate-regulated phosphoprotein 32 kDa is a crucial step for the induction of both long-term depression (LTD) and long-term potentiation (LTP), two opposing forms of synaptic plasticity. In addition, formation of LTD and LTP requires the activation of protein kinase G and protein kinase A, respectively, in striatal projection neurons. These kinases appear to be stimulated by the activation of D1-like receptors in distinct neuronal populations.
Figures





References
-
- Altar CA, Boyar WC, Kim HS. Discriminatory roles for D1 and D2 dopamine receptor subtypes in the in vivo control of neostriatal cyclic GMP. Eur J Pharmacol. 1990;181:17–21. - PubMed
-
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. - PubMed
-
- Calabresi P, Mercuri NB, Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons. II. An in vitro analysis. J Neurophysiol. 1990;63:663–675. - PubMed
-
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent block of NMDA receptor channels. Eur J Neurosci. 1992b;4:929–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
