Immunogenicity of an anti-clade B feline immunodeficiency fixed-cell virus vaccine in field cats
- PMID: 11069985
- PMCID: PMC113170
- DOI: 10.1128/jvi.74.23.10911-10919.2000
Immunogenicity of an anti-clade B feline immunodeficiency fixed-cell virus vaccine in field cats
Abstract
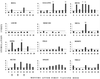
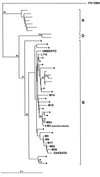
Attempts at vaccine development for feline immunodeficiency virus (FIV) have been extensive, both because this is a significant health problem for cats and because FIV may be a useful vaccine model for human immunodeficiency virus. To date, only modest success, producing only short-term protection, has been achieved for vaccine trials in controlled laboratory settings. It is unclear how relevant such experiments are to prevention of natural infection. The current study used a vaccine that employs cell-associated FIV-M2 strain fixed with paraformaldehyde. Subject cats were in a private shelter where FIV was endemic, a prevalence of 29 to 58% over an 8-year observation period. Cats roamed freely from the shelter through the surrounding countryside but returned for food and shelter. After ensuring that cats were FIV negative, they were immunized using six doses of vaccine over a 16-month period and observed for 28 months after the initiation of immunization. Twenty-six cats (12 immunized and 14 nonimmunized controls) were monitored for a minimum of 22 months. Immunized cats did not experience significant adverse effects from immunization and developed both antibodies and cellular immunity to FIV, although individual responses varied greatly. At the conclusion of the study, 0 of 12 immunized cats had evidence of FIV infection, while 5 of 14 control cats were infected. Thus, the vaccine was safe and immunogenic and did not transmit infection. Furthermore, vaccinated cats did not develop FIV infection in a limited clinical trial over an extended time period. Thus, the data suggest that a fixed, FIV-infected cell vaccine has potential for preventing natural FIV infection in free-roaming cats.
Figures



References
-
- Almond N M, Heeney J C. AIDS vaccine development in primate models. AIDS. 1998;12:S133–S144. - PubMed
-
- Bishop S A, Stokes C R, Gruffydd-Jones T J, Whiting C V, Humphries J E, Osborne R, Papanastasopoulou M, Harbour D A. Vaccination with fixed feline immunodeficiency virus (FIV) infected cells: protection, breakthrough and specificity of response. Vaccine. 1996;14:1243–1250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

