Characterization of human herpesvirus 8 ORF59 protein (PF-8) and mapping of the processivity and viral DNA polymerase-interacting domains
- PMID: 11069986
- PMCID: PMC113171
- DOI: 10.1128/jvi.74.23.10920-10929.2000
Characterization of human herpesvirus 8 ORF59 protein (PF-8) and mapping of the processivity and viral DNA polymerase-interacting domains
Abstract
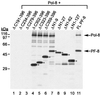
Human herpesvirus 8 (HHV-8) or Kaposi's sarcoma-associated herpesvirus (KSHV) ORF59 protein (PF-8) is a processivity factor for HHV-8 DNA polymerase (Pol-8) and is homologous to processivity factors expressed by other herpesviruses, such as herpes simplex virus type 1 UL42 and Epstein-Barr virus BMRF1. The interaction of UL42 and BMRF1 with their corresponding DNA polymerases is essential for viral DNA replication and the subsequent production of infectious virus. Using HHV-8-specific monoclonal antibody 11D1, we have previously identified the cDNA encoding PF-8 and showed that it is an early-late gene product localized to HHV-8-infected cell nuclei (S. R. Chan, C. Bloomer, and B. Chandran, Virology 240:118-126, 1998). Here, we have further characterized PF-8. This viral protein was phosphorylated both in vitro and in vivo. PF-8 bound double-stranded DNA (dsDNA) and single-stranded DNA independent of DNA sequence; however, the affinity for dsDNA was approximately fivefold higher. In coimmunoprecipitation reactions, PF-8 also interacted with Pol-8. In in vitro processivity assays with excess poly(dA):oligo(dT) as a template, PF-8 stimulated the production of elongated DNA products by Pol-8 in a dose-dependent manner. Functional domains of PF-8 were determined using PF-8 truncation mutants. The carboxyl-terminal 95 amino acids (aa) of PF-8 were dispensable for all three functions of PF-8: enhancing processivity of Pol-8, binding dsDNA, and binding Pol-8. Residues 10 to 27 and 279 to 301 were identified as regions critical for the processivity function of PF-8. Interestingly, aa 10 to 27 were also essential for binding Pol-8, whereas aa 1 to 62 and aa 279 to 301 were involved in binding dsDNA, suggesting that the processivity function of PF-8 is correlated with both the Pol-8-binding and the dsDNA-binding activities of PF-8.
Figures








References
-
- Ambroziak J A, Blackbourn D J, Herndier B G, Glogau R G, Gullett J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science. 1995;268:582–583. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987.
-
- Bambara R A, Fay P J, Mallaber L M. Methods of analyzing processivity. Methods Enzymol. 1995;262:270–280. - PubMed
-
- Beral V. Epidemiology of Kaposi's sarcoma. Cancer Surv. 1991;10:5–22. - PubMed
-
- Chan S R, Bloomer C, Chandran B. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology. 1998;240:118–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

