Hepatitis A virus-specific immunoglobulin A mediates infection of hepatocytes with hepatitis A virus via the asialoglycoprotein receptor
- PMID: 11069989
- PMCID: PMC113174
- DOI: 10.1128/jvi.74.23.10950-10957.2000
Hepatitis A virus-specific immunoglobulin A mediates infection of hepatocytes with hepatitis A virus via the asialoglycoprotein receptor
Abstract
The mechanisms underlying the hepatotropism of hepatitis A virus (HAV) and the relapsing courses of HAV infections are unknown. In this report, we show for a mouse hepatocyte model that HAV-specific immunoglobulin A (IgA) mediates infection of hepatocytes with HAV via the asialoglycoprotein receptor, which binds and internalizes IgA molecules. Proof of HAV infection was obtained by detection of HAV minus-strand RNA, which is indicative for virus replication, and quantification of infectious virions. We demonstrate that human hepatocytes also ingest HAV-anti-HAV IgA complexes by the same mechanism, resulting in infection of the cells, by using the HepG2 cell line and primary hepatocytes. The relevance of this surrogate receptor mechanism in HAV pathogenesis lies in the fact that HAV, IgA, and antigen-IgA complexes use the same pathway within the organism, leading from the gastrointestinal tract to the liver via blood and back to the gastrointestinal tract via bile fluid. Therefore, HAV-specific IgA antibodies produced by gastrointestinal mucosa-associated lymphoid tissue may serve as carrier and targeting molecules, enabling and supporting HAV infection of IgA receptor-positive hepatocytes and, in the case of relapsing courses, allowing reinfection of the liver in the presence of otherwise neutralizing antibodies, resulting in exacerbation of liver disease.
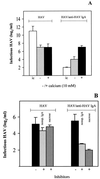
Figures




Similar articles
-
IgA-coated particles of Hepatitis A virus are translocalized antivectorially from the apical to the basolateral site of polarized epithelial cells via the polymeric immunoglobulin receptor.J Gen Virol. 2005 Oct;86(Pt 10):2747-2751. doi: 10.1099/vir.0.81157-0. J Gen Virol. 2005. PMID: 16186228
-
[Radioimmunological determination of HAV and anti-HAV IgA in the feces of patients with acute type-A hepatitis].Boll Ist Sieroter Milan. 1982;61(5):383-9. Boll Ist Sieroter Milan. 1982. PMID: 6100166 Italian.
-
The role of immunoglobulin A in prolonged and relapsing hepatitis A virus infections.J Gen Virol. 2012 Apr;93(Pt 4):754-760. doi: 10.1099/vir.0.038406-0. Epub 2011 Dec 14. J Gen Virol. 2012. PMID: 22170633
-
Immunologic approaches to assessing the response to inactivated hepatitis A vaccine.J Hepatol. 1993;18 Suppl 2:S15-9. doi: 10.1016/s0168-8278(05)80372-1. J Hepatol. 1993. PMID: 8182266 Review.
-
Passive immunization against hepatitis A.Vaccine. 1992;10 Suppl 1:S45-7. doi: 10.1016/0264-410x(92)90541-q. Vaccine. 1992. PMID: 1335658 Review.
Cited by
-
Hepatitis A virus inhibits cellular antiviral defense mechanisms induced by double-stranded RNA.J Virol. 2002 Dec;76(23):11920-30. doi: 10.1128/jvi.76.23.11920-11930.2002. J Virol. 2002. PMID: 12414934 Free PMC article.
-
Epitope structure of the carbohydrate recognition domain of asialoglycoprotein receptor to a monoclonal antibody revealed by high-resolution proteolytic excision mass spectrometry.J Am Soc Mass Spectrom. 2011 Jan;22(1):148-57. doi: 10.1007/s13361-010-0010-y. Epub 2011 Jan 20. J Am Soc Mass Spectrom. 2011. PMID: 21472553
-
Translation of Hepatitis A Virus IRES Is Upregulated by a Hepatic Cell-Specific Factor.Front Genet. 2018 Aug 10;9:307. doi: 10.3389/fgene.2018.00307. eCollection 2018. Front Genet. 2018. PMID: 30147706 Free PMC article.
-
A 'clicked' tetrameric hydroxamic acid glycopeptidomimetic antagonizes sugar-lectin interactions on the cellular level.Sci Rep. 2014 Jul 1;4:5513. doi: 10.1038/srep05513. Sci Rep. 2014. PMID: 24981800 Free PMC article.
-
Dynamic tracking of pathogenic receptor expression of live cells using pyrenyl glycoanthraquinone-decorated graphene electrodes.Chem Sci. 2015 Mar 1;6(3):1996-2001. doi: 10.1039/c4sc03614j. Epub 2015 Jan 13. Chem Sci. 2015. PMID: 28706649 Free PMC article.
References
-
- Asher L V, Binn L N, Mensing T L, Marchwicki R H, Vassell R A, Young G D. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus) J Med Virol. 1995;47:260–268. - PubMed
-
- Brown T A, Russel M W, Mestecky J. Elimination of intestinally absorbed antigen into the bile by IgA. J Immunol. 1984;132:780–782. - PubMed
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

