The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses
- PMID: 11069993
- PMCID: PMC113178
- DOI: 10.1128/jvi.74.23.10984-10993.2000
The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses
Abstract
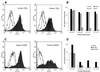
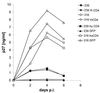
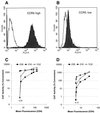
The entry of primate immunodeficiency viruses into cells is dependent on the interaction of the viral envelope glycoproteins with receptors, CD4, and specific members of the chemokine receptor family. Although in many cases the tropism of these viruses is explained by the qualitative pattern of coreceptor expression, several instances have been observed where the expression of a coreceptor on the cell surface is not sufficient to allow infection by a virus that successfully utilizes the coreceptor in a different context. For example, both the T-tropic simian immunodeficiency virus (SIV) SIVmac239 and the macrophagetropic (M-tropic) SIVmac316 can utilize CD4 and CCR5 as coreceptors, and both viruses can infect primary T lymphocytes, yet only SIVmac316 can efficiently infect CCR5-expressing primary macrophages from rhesus monkeys. Likewise, M-tropic strains of human immunodeficiency virus type 1 (HIV-1) do not infect primary rhesus monkey macrophages efficiently. Here we show that the basis of this restriction is the low level of CD4 on the surface of these cells. Overexpression of human or rhesus monkey CD4 in primary rhesus monkey macrophages allowed infection by both T-tropic and M-tropic SIV and by primary M-tropic HIV-1. By contrast, CCR5 overexpression did not specifically compensate for the inefficient infection of primary monkey macrophages by T-tropic SIV or M-tropic HIV-1. Apparently, the limited ability of these viruses to utilize a low density of CD4 for target cell entry accounts for the restriction of these viruses in primary rhesus monkey macrophages.
Figures
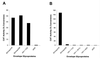
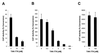
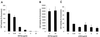
References
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Alkhatib G, Liao F, Berger E A, Farber J M, Peden K W. A new SIV co-receptor, STRL33. Nature. 1997;388:238. - PubMed
-
- Anderson M G, Hauer D, Sharma D P, Joag S V, Narayan O, Zink M C, Clements J E. Analysis of envelope changes acquired by SIVmac239 during neuroadaption in rhesus macaques. Virology. 1993;195:616–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

