The carboxy-terminal fragment of nucleolin interacts with the nucleocapsid domain of retroviral gag proteins and inhibits virion assembly
- PMID: 11069998
- PMCID: PMC113183
- DOI: 10.1128/jvi.74.23.11027-11039.2000
The carboxy-terminal fragment of nucleolin interacts with the nucleocapsid domain of retroviral gag proteins and inhibits virion assembly
Abstract
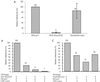
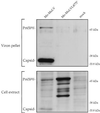
A yeast two-hybrid screen for cellular proteins that interact with the murine leukemia virus (MuLV) Gag protein resulted in the identification of nucleolin, a host protein known to function in ribosome assembly. The interacting fusions contained the carboxy-terminal 212 amino acids of nucleolin [Nuc(212)]. The nucleocapsid (NC) portion of Gag was necessary and sufficient to mediate the binding to Nuc(212). The interaction of Gag with Nuc(212) could be demonstrated in vitro and was manifested in vivo by the NC-dependent incorporation of Nuc(212) inside MuLV virions. Overexpression of Nuc(212), but not full-length nucleolin, potently and specifically blocked MuLV virion assembly and/or release. A mutant of MuLV, selected to specifically disrupt the binding to Nuc(212), was found to be severely defective for virion assembly. This mutant harbors a single point mutation in capsid (CA) adjacent to the CA-NC junction, suggesting a role for this region in Moloney MuLV assembly. These experiments demonstrate that selection for proteins that bind assembly domain(s) can yield potent inhibitors of virion assembly. These experiments also raise the possibility that a nucleolin-Gag interaction may be involved in virion assembly.
Figures






References
-
- Alin K, Goff S P. Amino acid substitutions in the CA protein of Moloney murine leukemia virus that block early events in infection. Virology. 1996;222:339–351. - PubMed
-
- Alin K, Goff S P. Mutational analysis of interactions between the Gag precursor proteins of murine leukemia viruses. Virology. 1996;216:418–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

