Lentivirus Nef specifically activates Pak2
- PMID: 11070003
- PMCID: PMC113188
- DOI: 10.1128/jvi.74.23.11081-11087.2000
Lentivirus Nef specifically activates Pak2
Abstract
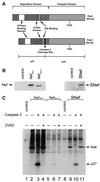
Nef proteins from human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) have been found to associate with an active cellular serine/threonine kinase designated Nef-associated kinase (Nak). The exact identity of Nak remains controversial, with two recent studies indicating that Nak may be either Pak1 or Pak2. In this study, we investigated the hypothesis that such discrepancies arise from the use of different Nef alleles or different cell types by individual investigators. We first confirm that Pak2 but not Pak1 is cleaved by caspase 3 in vitro and then demonstrate that Nak is caspase 3 sensitive, regardless of Nef allele or cell type used. We tested nef alleles from three lentiviruses (HIV-1 SF2, HIV-1 NL4-3, and SIVmac239) and used multiple cell lines of myeloid, lymphoid, and nonhematopoietic origin to evaluate the identity of Nak. We demonstrate that ectopically expressed Pak2 can substitute for Nak, while ectopically expressed Pak1 cannot. We then show that Nef specifically mediates the robust activation of ectopically expressed Pak2, directly demonstrating that Nef regulates Pak2 activity and does not merely associate with activated Pak2. We report that most of the active Pak2 is found bound to Nef, although a fraction is not. In contrast, only a small amount of Nef is found associated with Pak2. We conclude that Nak is Pak2 and that Nef specifically mediates Pak2 activation in a low-abundance complex. These results will facilitate both the elucidation of the role of Nef in pathogenesis and the development of specific inhibitors of this highly conserved function of Nef.
Figures
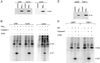
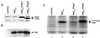
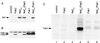
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

