DNA-Dependent protein kinase is not required for efficient lentivirus integration
- PMID: 11070027
- PMCID: PMC113232
- DOI: 10.1128/jvi.74.23.11278-11285.2000
DNA-Dependent protein kinase is not required for efficient lentivirus integration
Abstract
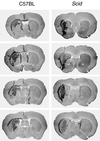
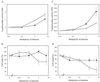
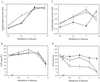
How DNA is repaired after retrovirus integration is not well understood. DNA-dependent protein kinase (DNA-PK) is known to play a central role in the repair of double-stranded DNA breaks. Recently, a role for DNA-PK in retroviral DNA integration has been proposed (R. Daniel, R. A. Katz, and A. M. Skalka, Science 284:644-647, 1999). Reduced transduction efficiency and increased cell death by apoptosis were observed upon retrovirus infection of cultured scid cells. We have used a human immunodeficiency virus (HIV) type 1 (HIV-1)-derived lentivirus vector system to further investigate the role of DNA-PK during integration. We measured lentivirus transduction of scid mouse embryonic fibroblasts (MEF) and xrs-5 or xrs-6 cells. These cells are deficient in the catalytic subunit of DNA-PK and in Ku, the DNA-binding subunit of DNA-PK, respectively. At low vector titers, efficient and stable lentivirus transduction was obtained, excluding an essential role for DNA-PK in lentivirus integration. Likewise, the efficiency of transduction of HIV-derived vectors in scid mouse brain was as efficient as that in control mice, without evidence of apoptosis. We observed increased cell death in scid MEF and xrs-5 or xrs-6 cells, but only after transduction with high vector titers (multiplicity of infection [MOI], >1 transducing unit [TU]/cell) and subsequent passage of the transduced cells. At an MOI of <1 TU/cell, however, transduction efficiency was even higher in DNA-PK-deficient cells than in control cells. Taken together, the data suggest a protective role of DNA-PK against cellular toxicity induced by high levels of retrovirus integrase or integration. Another candidate cellular enzyme that has been claimed to play an important role during retrovirus integration is poly(ADP-ribose) polymerase (PARP). However, no inhibition of lentivirus vector-mediated transduction or HIV-1 replication by 3-methoxybenzamide, a known PARP inhibitor, was observed. In conclusion, DNA-PK and PARP are not essential for lentivirus integration.
Figures




References
-
- Abdallah B, Hassan A, Benoist C, Goula D, Behr J P, Demeneix B A. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther. 1997;7:1947–1954. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987.
-
- Baekelandt, V., B. De Strooper, B. Nuttin, and Z. Debyser. Gene therapeutic strategies for neurodegenerative diseases. Curr. Opin. Mol. Ther., in press. - PubMed
-
- Blier P R, Griffith A J, Craft J, Hardin J A. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J Biol Chem. 1993;268:7594–7601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

