Interaction between herpes simplex virus type 1 IE63 protein and cellular protein p32
- PMID: 11070032
- PMCID: PMC113237
- DOI: 10.1128/jvi.74.23.11322-11328.2000
Interaction between herpes simplex virus type 1 IE63 protein and cellular protein p32
Abstract
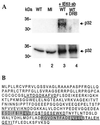
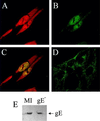
The herpes simplex virus type 1 (HSV-1) immediate-early gene IE63 (ICP27), the only HSV-1 regulatory gene with a homologue in every mammalian and avian herpesvirus sequenced so far, is a multifunctional protein which regulates transcriptional and posttranscriptional processes. One of its posttranscriptional effects is the inhibition of splicing of viral and cellular transcripts. We previously identified heterogeneous nuclear ribonucleoprotein (hnRNP) K and casein kinase 2 (CK2) as two protein partners of IE63 (H. Bryant et al., J. Biol. Chem. 274:28991-28998, 1999). Here, using a yeast two-hybrid assay, we identify another partner of IE63, the cellular protein p32. Confirmation of this interaction was provided by coimmunoprecipitation from virus-infected cells and recombinant p32 binding assays. A p32-hnRNP K-CK2 complex, which required IE63 to form, was isolated from HSV-1-infected cells, and coimmunoprecipitating p32 was phosphorylated by CK2. Expression of IE63 altered the cytoplasmic distribution of p32, with some now colocalizing with IE63 in the nuclei of infected and transfected cells. As p32 copurifies with splicing factors and can inhibit splicing, we propose that IE63 together with p32, possibly with other IE63 partner proteins, acts to disrupt or regulate pre-mRNA splicing. As well as contributing to host cell shutoff, this effect could facilitate splicing-independent nuclear export of viral transcripts.
Figures



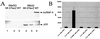
References
-
- Bryant H, Wadd S, Filhol O, Scott J E, Hsieh T, Everett R, Clements J B. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J Biol Chem. 1999;274:28991–28998. - PubMed
-
- Chen M R, Yang J F, Wu C W, Middeldorp J M, Chen J Y. Physical association between the EBV protein EBNA 1 and P32/TAP/hyaluronectin. J Biomed Sci. 1998;5:173–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

