The host range phenotype displayed by a Sindbis virus glycoprotein variant results from virion aggregation and retention on the surface of mosquito cells
- PMID: 11070041
- PMCID: PMC113246
- DOI: 10.1128/jvi.74.23.11398-11406.2000
The host range phenotype displayed by a Sindbis virus glycoprotein variant results from virion aggregation and retention on the surface of mosquito cells
Abstract
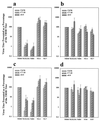
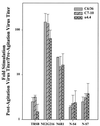
The Sindbis virus variant NE2G216 is a PE2-containing host range mutant that is growth restricted in cultured mosquito cells (C6/36) due to inefficient release of virions from this cell type. The maturation defect of NE2G216 has been linked to the structures of N-linked oligosaccharides synthesized by arthropod cells. Analysis of C6/36 cells infected with NE2G216 by transmission electron microscopy revealed the presence of dense virus aggregates within cytoplasmic vacuoles and virus aggregates adhered to the cell surface. The virus aggregation phenotype of NE2G216 was reproduced in vertebrate cells (Pro-5) by the addition of 1-deoxymannojirimycin, an inhibitor of carbohydrate processing which limits the processing of N-linked oligosaccharides to structures that are structurally similar, albeit not identical, to those synthesized in C6/36 cells. We conclude that defective maturation of NE2G216 in mosquito cells is due to virion aggregation and retention on the cell surface and that this phenotype is directly linked to the carbohydrate-processing properties of these cells.
Figures



References
-
- Bischoff J, Liscum L, Kornfeld R. The use of 1-deoxymannojirimycin to evaluate the role of various α-mannosidases in oligosaccharide processing in intact cells. J Biol Chem. 1986;261:4766–4774. - PubMed
-
- Boehme K W, Williams J C, Johnston R E, Heidner H W. Linkage of an alphavirus host-range restriction to the carbohydrate processing phenotypes of the host cell. J Gen Virol. 2000;81:161–170. - PubMed
-
- Burge B W, Pfefferkorn E R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966;30:204–213. - PubMed
-
- Doms R W, Lamb R A, Rose J K, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. - PubMed
-
- Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

