Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy
- PMID: 11070088
- PMCID: PMC18835
- DOI: 10.1073/pnas.97.23.12746
Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy
Abstract
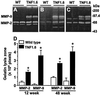
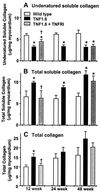
Myocardial fibrosis caused by maladaptive extracellular matrix (ECM) remodeling is implicated in the dysfunction of the failing heart. Matrix metalloproteinases (MMPs) regulate ECM remodeling, and are regulated by cytokines. Transgenic mice with cardiac-specific overexpression of tumor necrosis factor alpha (TNF-alpha) (TNF1.6) develop heart failure. We hypothesized that modulation of TNF-alpha and/or MMP activity might alter the myocardial ECM remodeling process and the development of heart failure. To test this hypothesis, we took advantage of the TNF1.6 mice and studied soluble and total collagens and collagen type profiling by using hydroxyproline quantification, Sircol collagen assay, Northern blot analysis, and immunohistochemistry and studied myocardial function by using echocardiography. Progressive ventricular hypertrophy and dilation in the TNF1.6 mice were accompanied by a significant increase in MMP-2 and MMP-9 activity, an increase in collagen synthesis, deposition, and denaturation, and a decrease in undenatured collagens. In young TNF1.6 mice, these changes in the ECM were associated with marked diastolic dysfunction as demonstrated by significantly reduced transmitral Doppler echocardiographic E/A wave ratio. Anti-TNF-alpha treatment with adenoviral vector expressing soluble TNF-alpha receptor type I attenuated both MMP-2 and MMP-9 activity, prevented further collagen synthesis, deposition and denaturation, and preserved myocardial diastolic function in young, but not old, TNF1.6 mice. The results suggest a critical role of TNF-alpha and MMPs in myocardial matrix remodeling and functional regulation and support the hypothesis that both TNF-alpha and MMPs may serve as potential therapeutic targets in the treatment of heart failure.
Figures




References
-
- Weber K T, Brilla C G, Campbell S E, Zhou G, Matsubara L, Guarda E. Blood Pressure. 1992;1:75–85. - PubMed
-
- Weber K T, Pick R, Jalil J E, Janicki J S, Carroll E P. J Mol Cell Cardiol. 1989;21, Suppl. 5:121–131. - PubMed
-
- Weber K T. J Am Coll Cardiol. 1989;13:1637–1652. - PubMed
-
- Jalil J E, Doering C W, Janicki J S, Pick R, Shroff S G, Weber K T. Circ Res. 1989;64:1041–1050. - PubMed
-
- Mukherjee D, Sen S. Circ Res. 1990;67:1474–1480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

