Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas
- PMID: 11070094
- PMCID: PMC18852
- DOI: 10.1073/pnas.97.23.12846
Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas
Erratum in
- Proc Natl Acad Sci U S A 2001 Jan 16;98(2):777
Abstract
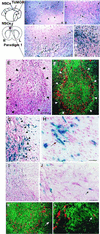
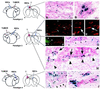
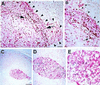
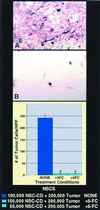
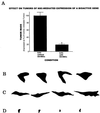
One of the impediments to the treatment of brain tumors (e.g., gliomas) has been the degree to which they expand, infiltrate surrounding tissue, and migrate widely into normal brain, usually rendering them "elusive" to effective resection, irradiation, chemotherapy, or gene therapy. We demonstrate that neural stem cells (NSCs), when implanted into experimental intracranial gliomas in vivo in adult rodents, distribute themselves quickly and extensively throughout the tumor bed and migrate uniquely in juxtaposition to widely expanding and aggressively advancing tumor cells, while continuing to stably express a foreign gene. The NSCs "surround" the invading tumor border while "chasing down" infiltrating tumor cells. When implanted intracranially at distant sites from the tumor (e.g., into normal tissue, into the contralateral hemisphere, or into the cerebral ventricles), the donor cells migrate through normal tissue targeting the tumor cells (including human glioblastomas). When implanted outside the CNS intravascularly, NSCs will target an intracranial tumor. NSCs can deliver a therapeutically relevant molecule-cytosine deaminase-such that quantifiable reduction in tumor burden results. These data suggest the adjunctive use of inherently migratory NSCs as a delivery vehicle for targeting therapeutic genes and vectors to refractory, migratory, invasive brain tumors. More broadly, they suggest that NSC migration can be extensive, even in the adult brain and along nonstereotypical routes, if pathology (as modeled here by tumor) is present.
Figures

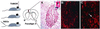
Comment in
-
Are denatured proteins ever random coils?Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12391-2. doi: 10.1073/pnas.97.23.12391. Proc Natl Acad Sci U S A. 2000. PMID: 11070072 Free PMC article. Review. No abstract available.
-
Can neural stem cells be used to track down and destroy migratory brain tumor cells while also providing a means of repairing tumor-associated damage?Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12393-5. doi: 10.1073/pnas.97.23.12393. Proc Natl Acad Sci U S A. 2000. PMID: 11070073 Free PMC article. Review. No abstract available.
References
-
- Black P M, Loeffler J, editors. Cancer of the Nervous System. Oxford: Blackwell; 1997.
-
- Kramm C M, Sena-Esteves M, Barnett F H, Rainov N G, Schuback D E, Yu J S, Pechan P A, Paulus W, Chiocca E A, Breakefield X O. Brain Pathol. 1995;5:345–381. - PubMed
-
- Gage F H. Science. 2000;287:1433–1438. - PubMed
-
- McKay R. Science. 1997;276:66–71. - PubMed
-
- Flax J D, Aurora S, Yang C, Simonin C, Wills A M, Billinghurst M J, Sidman R L, Wolfe J H, Kim S U, Snyder E Y. Nat Biotech. 1998;16:1033–1039. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

