The multimerization of human immunodeficiency virus type I Vif protein: a requirement for Vif function in the viral life cycle
- PMID: 11071884
- PMCID: PMC1350968
- DOI: 10.1074/jbc.M004895200
The multimerization of human immunodeficiency virus type I Vif protein: a requirement for Vif function in the viral life cycle
Abstract
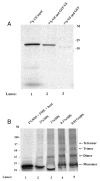
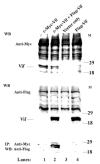
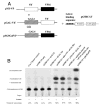
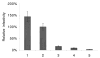
The Vif (virion infectivity factor protein of human immunodeficiency virus type I (HIV-1) is essential for viral replication in vivo and productive infection of peripheral blood mononuclear cells, macrophages, and H9 T-cells. However, the molecular mechanism(s) of Vif remains unknown and needs to be further determined. In this report, we show that, like many other proteins encoded by HIV-1, Vif proteins possess a strong tendency toward self-association. In relatively native conditions, Vif proteins formed multimers in vitro, including dimers, trimers, or tetramers. Through in vivo binding assays such as coimmunoprecipitation and the mammalian two-hybrid system, we also demonstrated that Vif proteins could interact with each other within a cell, indicating that the multimerization of Vif proteins is not simply due to fortuitous aggregation. Further studies indicated that the domain affecting Vif self-association is located at the C terminus of this protein, especially the proline-enriched 151-164 region. Moreover, we found that a Vif mutant with deletion at amino acid 151-164 was unable to rescue the infectivity of vif-defective viruses generated from H9 T-cells, suggesting that the multimerization of Vif proteins could be important for Vif function in the viral life cycle. Our studies identified a new feature of Vif and should accelerate our understanding of its role in HIV-1 pathogenesis.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

