Mechanisms of apoptosis
- PMID: 11073801
- PMCID: PMC1885741
- DOI: 10.1016/S0002-9440(10)64779-7
Mechanisms of apoptosis
Abstract
Programmed cell death plays critical roles in a wide variety of physiological processes during fetal development and in adult tissues. In most cases, physiological cell death occurs by apoptosis as opposed to necrosis. Defects in apoptotic cell death regulation contribute to many diseases, including disorders where cell accumulation occurs (cancer, restenosis) or where cell loss ensues (stroke, heart failure, neurodegeneration, AIDS). In recent years, the molecular machinery responsible for apoptosis has been elucidated, revealing a family of intracellular proteases, the caspases, which are responsible directly or indirectly for the morphological and biochemical changes that characterize the phenomenon of apoptosis. Diverse regulators of the caspases have also been discovered, including activators and inhibitors of these cell death proteases. Inputs from signal transduction pathways into the core of the cell death machinery have also been identified, demonstrating ways of linking environmental stimuli to cell death responses or cell survival maintenance. Knowledge of the molecular mechanisms of apoptosis is providing insights into the causes of multiple pathologies where aberrant cell death regulation occurs and is beginning to provide new approaches to the treatment of human diseases.
Figures



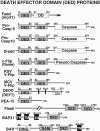



References
-
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J: Human ICE/CED-3 protease nomenclature. Cell 1996, 87:171. - PubMed
-
- Thornberry N, Lazebnik Y: Caspases: enemies within. Science 1998, 281:1312-1316 - PubMed
-
- Cryns V, Yuan Y: Proteases to die for. Genes Dev 1999, 13:371 - PubMed
-
- Nicholson DW: ICE/CED3-like proteases as therapeutic targets for the control of inappropriate apoptosis. Nat Biotechnol 1996, 14:297-301 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

