Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples
- PMID: 11073807
- PMCID: PMC1885742
- DOI: 10.1016/S0002-9440(10)64785-2
Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples
Abstract
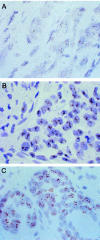
Determination of HER-2/neu oncogene amplification has become necessary for selection of breast cancer patients for trastuzumab (Herceptin) therapy. Fluorescence in situ hybridization (FISH) is currently regarded as a gold standard method for detecting HER-2/neu amplification, but it is not very practical for routine histopathological laboratories. We evaluated a new modification of in situ hybridization, the chromogenic in situ hybridization (CISH), which enables detection of HER-2/neu gene copies with conventional peroxidase reaction. Archival formalin-fixed paraffin-embedded tumor tissue sections were pretreated (by heating in a microwave oven and using enzyme digestion) and hybridized with a digoxigenin-labeled DNA probe. The probe was detected with anti-digoxigenin fluorescein, anti-fluorescein peroxidase, and diaminobenzidine. Gene copies visualized by CISH could be easily distinguished with a x40 objective in hematoxylin-stained tissue sections. HER-2/neu amplification typically appeared as large peroxidase-positive intranuclear gene copy clusters. CISH and FISH (according to Vysis, made from frozen pulverized tumor samples) correlated well in a series of 157 breast cancers (kappa coefficient, 0.81). The few different classifications were mostly because of low-level amplifications by FISH that were negative by CISH and immunohistochemistry with monoclonal antibody CB-11. We conclude that CISH, using conventional bright-field microscopy in evaluation, is a useful alternative for determination of HER-2/neu amplification in paraffin-embedded tumor samples, especially for confirming the immunohistochemical staining results.
Figures
References
-
- Ross JS, Fletcher JA: HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol 1999, 112:S53-S67 - PubMed
-
- Shak S: Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Herceptin Multinational Investigator Study Group. Semin Oncol 1999, 6:71-77 - PubMed
-
- Press MF, Hung G, Godolphin W, Slamon DJ: Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res 1994, 54:2771-2777 - PubMed
-
- Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ: Specificity of Hercep Test in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol 1999, 17:1983. - PubMed
-
- Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ: Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancer. J Clin Oncol 1999, 17:1974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

