Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord
- PMID: 11073810
- PMCID: PMC1885744
- DOI: 10.1016/S0002-9440(10)64788-8
Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord
Abstract
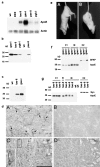
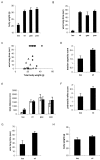
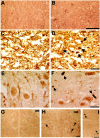
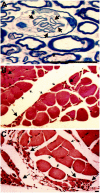
The epsilon 4 allele of the human apolipoprotein E gene (ApoE4) constitutes an important genetic risk factor for Alzheimer's disease. Recent experimental evidence suggests that human ApoE is expressed in neurons, in addition to being synthesized in glial cells. Moreover, brain regions in which neurons express ApoE seem to be most vulnerable to neurofibrillary pathology. The hypothesis that the expression pattern of human ApoE might be important for the pathogenesis of Alzheimer's disease was tested by generating transgenic mice that express human ApoE4 in neurons or in astrocytes of the central nervous system. Transgenic mice expressing human ApoE4 in neurons developed axonal degeneration and gliosis in brain and in spinal cord, resulting in reduced sensorimotor capacities. In these mice, axonal dilatations with accumulation of synaptophysin, neurofilaments, mitochondria, and vesicles were documented, suggesting impairment of axonal transport. In contrast, transgenic mice expressing human ApoE4 in astrocytes remained normal throughout life. These results suggest that expression of human ApoE in neurons of the central nervous system could contribute to impaired axonal transport and axonal degeneration. The possible contribution of hyperphosphorylation of protein Tau to the resulting phenotype is discussed.
Figures


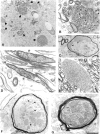

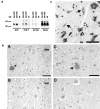
References
-
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families [see comments]. Science 1993, 261:921-923 - PubMed
-
- Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M, Schmechel D, Roses AD: Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci USA 1994, 91:11183-11186 - PMC - PubMed
-
- Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, Thal LJ: The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology 1996, 47:190-196 - PubMed
-
- Nagy Z, Esiri MM, Jobst KA, Johnston C, Litchfield S, Sim E, Smith AD: Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease. Neuroscience 1995, 69:757-761 - PubMed
-
- Gearing M, Mori H, Mirra SS: A beta-peptide length and apolipoprotein E genotype in Alzheimer’s disease. Ann Neurol 1996, 39:395-399 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

