Keloid-derived fibroblasts are refractory to Fas-mediated apoptosis and neutralization of autocrine transforming growth factor-beta1 can abrogate this resistance
- PMID: 11073825
- PMCID: PMC1885731
- DOI: 10.1016/s0002-9440(10)64803-1
Keloid-derived fibroblasts are refractory to Fas-mediated apoptosis and neutralization of autocrine transforming growth factor-beta1 can abrogate this resistance
Abstract
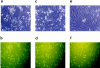
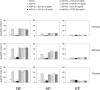
The pathogenesis of keloid remains poorly understood. As no effective therapy for keloid is as yet available, an insight into its pathogenesis may lead to novel approaches. Apoptosis has been found to mediate the decrease in cellularity during the transition between granulation tissue and scar. Here, we report that in contrast to hypertrophic scar-derived and normal skin-derived fibroblasts, keloid-derived fibroblasts are significantly resistant to both Fas-mediated and staurosporine-induced apoptosis. The caspases-3, -8, and -9 were not activated indicating that the block in the apoptotic pathway in keloid is upstream of the caspases. There were no significant differences in the level of expression of Fas, Bcl-2, and Bax between the three groups but addition of transforming growth factor (TGF)-beta1 significantly inhibited Fas-mediated apoptosis in hypertrophic scar-derived and normal skin-derived fibroblasts and neutralization of autocrine TGF-beta1 with anti-TGF-beta1 antibody abrogated the resistance of keloid-derived fibroblasts. Anti-apoptotic activity was not observed with TGF-beta2. This is the first study linking refractory Fas-mediated apoptosis to cellular phenotype in keloids and indicating a pivotal role for the anti-apoptotic effect of TGF-beta1 in this resistance. Hence, it becomes important to treat keloids as a separate entity different from hypertrophic scars and enhancement of Fas-sensitivity could be a promising therapeutic target.
Figures





References
-
- Alhady S: Keloids in various races. Plast Reconstr Surg 1969, 44:564-566 - PubMed
-
- Murray JC, Pollack SV, Pinnelli SR: Keloids: a review. J Am Acad Dermatol 1981, 4:461-470 - PubMed
-
- Ladin DA, Garner WL, Smith DJ: Excessive scarring as a consequence of healing. Wound Rep Reg 1995, 3:6-14 - PubMed
-
- Nicoletis C, Bazin S, LeLous M: Clinical and biochemical features of normal, defective and pathologic scars. Clin Plast Surg 1977, 4:347-359 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

