Regulation of the functional interaction between cyclin D1 and the estrogen receptor
- PMID: 11073968
- PMCID: PMC86475
- DOI: 10.1128/MCB.20.23.8667-8675.2000
Regulation of the functional interaction between cyclin D1 and the estrogen receptor
Abstract
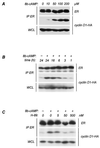
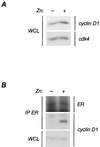
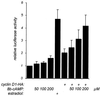
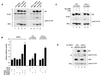
We report that the functional interaction between cyclin D1 and the estrogen receptor (ER) is regulated by a signal transduction pathway involving the second messenger, cyclic AMP (cAMP). The cell-permeable cAMP analogue 8-bromo-cAMP caused a concentration-dependent enhancement of cyclin D1-ER complex formation, as judged both by coimmunoprecipitation and mammalian two-hybrid analysis. This effect was paralleled by increases in ligand-independent ER-mediated transcription from an estrogen response element containing reporter construct. These effects of 8-bromo-cAMP were antagonized by a specific protein kinase A (PKA) inhibitor, indicating that the signaling pathway involved was PKA dependent. Further, we show that culture of MCF-7 cells on a cellular substratum of murine preadipocytes also enhanced the functional interaction between cyclin D1 and ER in a PKA-dependent manner. These findings demonstrate a collaboration between cAMP signaling and cyclin D1 in the ligand-independent activation of ER-mediated transcription in mammary epithelial cells and show that the functional associations of cyclin D1 are regulated as a function of cellular context.
Figures

References
-
- Aggeler J, Ward J, Blackie L M, Barcellos-Hoff M H, Streuli C H, Bissell M J. Cytodifferentiation of mouse mammary epithelial cells cultured on reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci. 1991;99:407–417. - PubMed
-
- Aronica S M, Katzenellenbogen B S. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-1. Mol Endocrinol. 1993;7:743–752. - PubMed
-
- Barcellos-Hoff M H. Mammary epithelial reorganization on extracellular matrix is mediated by cell surface galactosyltransferase. Exp Cell Res. 1992;201:225–234. - PubMed
-
- Bartkova J, Lukas J, Muller H, Luzhoft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57:353–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
