Association with Ets-1 causes ligand- and AF2-independent activation of nuclear receptors
- PMID: 11073980
- PMCID: PMC86515
- DOI: 10.1128/MCB.20.23.8793-8802.2000
Association with Ets-1 causes ligand- and AF2-independent activation of nuclear receptors
Abstract
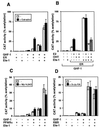
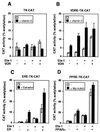
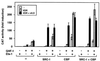
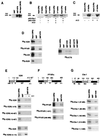
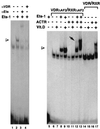
The vitamin D receptor (VDR) normally functions as a ligand-dependent transcriptional activator. Here we show that, in the presence of Ets-1, VDR stimulates the prolactin promoter in a ligand-independent manner, behaving as a constitutive activator. Mutations in the AF2 domain abolish vitamin D-dependent transactivation but do not affect constitutive activation by Ets-1. Therefore, in contrast with the actions of vitamin D, activation by Ets-1 is independent of the AF2 domain. Ets-1 also conferred a ligand-independent activation to the estrogen receptor and to peroxisome proliferator-activated receptor alpha. In addition, Ets-1 cooperated with the unliganded receptors to stimulate the activity of reporter constructs containing consensus response elements fused to the thymidine kinase promoter. There is a direct interaction of the receptors with Ets-1 which requires the DNA binding domains of both proteins. Interaction with Ets-1 induces a conformational change in VDR which can be detected by an increased resistance to proteolytic digestion. Furthermore, a retinoid X receptor-VDR heterodimer in which both receptors lack the core C-terminal AF2 domain can recruit coactivators in the presence, but not in the absence, of Ets-1. This suggests that Ets-1 induces a conformational change in the receptor which creates an active interaction surface with coactivators even in the AF2-defective mutants. These results demonstrate the existence of a novel mechanism, alternative to ligand binding, which can convert an unliganded receptor from an inactive state into a competent transcriptional activator.
Figures
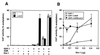

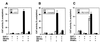


References
-
- Castillo A I, Tolon R M, Aranda A. Insulin-like growth factor-1 stimulates rat prolactin gene expression by a Ras, ETS and phosphatidylinositol 3-kinase dependent mechanism. Oncogene. 1998;16:1981–1991. - PubMed
-
- Castillo A I, Jiménez-Lara A M, Tolon R M, Aranda A. Synergistic activation of the prolactin promoter by vitamin D and GHF-1: role of the coactivators CBP and SRC-1. Mol Endocrinol. 1999;13:1141–1154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
