A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex
- PMID: 11073988
- PMCID: PMC86543
- DOI: 10.1128/MCB.20.23.8879-8888.2000
A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex
Abstract
The SWI/SNF family of chromatin-remodeling complexes facilitates gene activation by assisting transcription machinery to gain access to targets in chromatin. This family includes BAF (also called hSWI/SNF-A) and PBAF (hSWI/SNF-B) from humans and SWI/SNF and Rsc from Saccharomyces cerevisiae. However, the relationship between the human and yeast complexes is unclear because all human subunits published to date are similar to those of both yeast SWI/SNF and Rsc. Also, the two human complexes have many identical subunits, making it difficult to distinguish their structures or functions. Here we describe the cloning and characterization of BAF250, a subunit present in human BAF but not PBAF. BAF250 contains structural motifs conserved in yeast SWI1 but not in any Rsc components, suggesting that BAF is related to SWI/SNF. BAF250 is also a homolog of the Drosophila melanogaster Osa protein, which has been shown to interact with a SWI/SNF-like complex in flies. BAF250 possesses at least two conserved domains that could be important for its function. First, it has an AT-rich DNA interaction-type DNA-binding domain, which can specifically bind a DNA sequence known to be recognized by a SWI/SNF family-related complex at the beta-globin locus. Second, BAF250 stimulates glucocorticoid receptor-dependent transcriptional activation, and the stimulation is sharply reduced when the C-terminal region of BAF250 is deleted. This region of BAF250 is capable of interacting directly with the glucocorticoid receptor in vitro. Our data suggest that BAF250 confers specificity to the human BAF complex and may recruit the complex to its targets through either protein-DNA or protein-protein interactions.
Figures
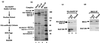






References
-
- Armstrong J A, Bieker J J, Emerson B M. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell. 1998;95:93–104. - PubMed
-
- Cairns B R, Erdjument-Bromage H, Tempst P, Winston F, Kornberg R D. Two actin-related proteins are shared functional components of the chromatin-remodeling complexes RSC and SWI/SNF. Mol Cell. 1998;2:639–651. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
