Rdp1, a novel zinc finger protein, regulates the DNA damage response of rhp51(+) from Schizosaccharomyces pombe
- PMID: 11073995
- PMCID: PMC86550
- DOI: 10.1128/MCB.20.23.8958-8968.2000
Rdp1, a novel zinc finger protein, regulates the DNA damage response of rhp51(+) from Schizosaccharomyces pombe
Abstract
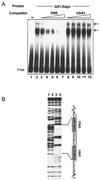
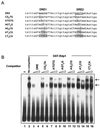
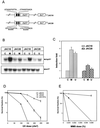
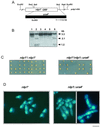
The Schizosaccharomyces pombe DNA repair gene rhp51(+) encodes a RecA-like protein with the DNA-dependent ATPase activity required for homologous recombination. The level of the rhp51(+) transcript is increased by a variety of DNA-damaging agents. Its promoter has two cis-acting DNA damage-responsive elements (DREs) responsible for DNA damage inducibility. Here we report identification of Rdp1, which regulates rhp51(+) expression through the DRE of rhp51(+). The protein contains a zinc finger and a polyalanine tract similar to ones previously implicated in DNA binding and transactivation or repression, respectively. In vitro footprinting and competitive binding assays indicate that the core consensus sequences (NGG/TTG/A) of DRE are crucial for the binding of Rdp1. Mutations of both DRE1 and DRE2 affected the damage-induced expression of rhp51(+), indicating that both DREs are required for transcriptional activation. In addition, mutations in the DREs significantly reduced survival rates after exposure to DNA-damaging agents, demonstrating that the damage response of rhp51(+) enhances the cellular repair capacity. Surprisingly, haploid cells containing a complete rdp1 deletion could not be recovered, indicating that rdp1(+) is essential for cell viability and implying the existence of other target genes. Furthermore, the DNA damage-dependent expression of rhp51(+) was significantly reduced in checkpoint mutants, raising the possibility that Rdp1 may mediate damage checkpoint-dependent transcription of rhp51(+).
Figures


Similar articles
-
Identification of the DNA damage-responsive elements of the rhp51+ gene, a recA and RAD51 homolog from the fission yeast Schizosaccharomyces pombe.Mol Gen Genet. 1996 May 23;251(2):167-75. doi: 10.1007/BF02172915. Mol Gen Genet. 1996. PMID: 8668127
-
Cloning and sequence analysis of rhp51+, a Schizosaccharomyces pombe homolog of the Saccharomyces cerevisiae RAD51 gene.Gene. 1994 May 16;142(2):207-11. doi: 10.1016/0378-1119(94)90262-3. Gene. 1994. PMID: 8194753
-
Gain- and loss-of-function of Rhp51, a Rad51 homolog in fission yeast, reveals dissimilarities in chromosome integrity.Nucleic Acids Res. 2001 Apr 15;29(8):1724-32. doi: 10.1093/nar/29.8.1724. Nucleic Acids Res. 2001. PMID: 11292845 Free PMC article.
-
Cloning the RAD51 homologue of Schizosaccharomyces pombe.Nucleic Acids Res. 1993 Sep 25;21(19):4586-91. doi: 10.1093/nar/21.19.4586. Nucleic Acids Res. 1993. PMID: 8233794 Free PMC article.
-
Molecular biology of DNA repair in the fission yeast Schizosaccharomyces pombe.Mutat Res. 1996 Aug 8;363(3):147-61. doi: 10.1016/0921-8777(96)00017-1. Mutat Res. 1996. PMID: 8765156 Review. No abstract available.
Cited by
-
Homologous recombination is essential for RAD51 up-regulation in Saccharomyces cerevisiae following DNA crosslinking damage.Nucleic Acids Res. 2002 Mar 1;30(5):1224-32. doi: 10.1093/nar/30.5.1224. Nucleic Acids Res. 2002. PMID: 11861915 Free PMC article.
-
Global gene expression responses of fission yeast to ionizing radiation.Mol Biol Cell. 2004 Feb;15(2):851-60. doi: 10.1091/mbc.e03-08-0569. Epub 2003 Dec 10. Mol Biol Cell. 2004. PMID: 14668484 Free PMC article.
-
A composite DNA element that functions as a maintainer required for epigenetic inheritance of heterochromatin.Mol Cell. 2021 Oct 7;81(19):3979-3991.e4. doi: 10.1016/j.molcel.2021.07.017. Epub 2021 Aug 9. Mol Cell. 2021. PMID: 34375584 Free PMC article.
-
The MluI cell cycle box (MCB) motifs, but not damage-responsive elements (DREs), are responsible for the transcriptional induction of the rhp51+ gene in response to DNA replication stress.PLoS One. 2014 Nov 5;9(11):e111936. doi: 10.1371/journal.pone.0111936. eCollection 2014. PLoS One. 2014. PMID: 25372384 Free PMC article.
-
UV irradiation causes the loss of viable mitotic recombinants in Schizosaccharomyces pombe cells lacking the G(2)/M DNA damage checkpoint.Genetics. 2002 Mar;160(3):891-908. doi: 10.1093/genetics/160.3.891. Genetics. 2002. PMID: 11901109 Free PMC article.
References
-
- Aruga J, Nagai T, Tokuyama T, Hayashizaki Y, Okazaki Y, Chapman V M, Mikoshiba K. The mouse zic gene family. Homologues of the Drosophila pair-rule gene odd-paired. J Biol Chem. 1996;271:1043–1047. - PubMed
-
- Bang D D, Ketting R, Ruijter M, Brandsma J A, Verhage R A, Putte P, Brouwer J. Cloning of Schizosaccharomyces pombe rhp16+, a gene homologous to the Saccharomyces cerevisiae RAD16 gene. Mutat Res. 1996;364:57–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
