TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture
- PMID: 11073998
- PMCID: PMC86553
- DOI: 10.1128/MCB.20.23.8996-9008.2000
TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture
Abstract
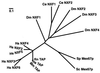
Vertebrate TAP (also called NXF1) and its yeast orthologue, Mex67p, have been implicated in the export of mRNAs from the nucleus. The TAP protein includes a noncanonical RNP-type RNA binding domain, four leucine-rich repeats, an NTF2-like domain that allows heterodimerization with p15 (also called NXT1), and a ubiquitin-associated domain that mediates the interaction with nucleoporins. Here we show that TAP belongs to an evolutionarily conserved family of proteins that has more than one member in higher eukaryotes. Not only the overall domain organization but also residues important for p15 and nucleoporin interaction are conserved in most family members. We characterize two of four human TAP homologues and show that one of them, NXF2, binds RNA, localizes to the nuclear envelope, and exhibits RNA export activity. NXF3, which does not bind RNA or localize to the nuclear rim, has no RNA export activity. Database searches revealed that although only one p15 (nxt) gene is present in the Drosophila melanogaster and Caenorhabditis elegans genomes, there is at least one additional p15 homologue (p15-2 [also called NXT2]) encoded by the human genome. Both human p15 homologues bind TAP, NXF2, and NXF3. Together, our results indicate that the TAP-p15 mRNA export pathway has diversified in higher eukaryotes compared to yeast, perhaps reflecting a greater substrate complexity.
Figures




References
-
- Adams M D, Celniker S E, Holt R A, Evans C A, Gocayne J D, Amanatides P G, Scherer S E, Li P W, Hoskins R A, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
